DOI:
10.1039/C5RA26641F
(Paper)
RSC Adv., 2016,
6, 19351-19356
High transparent alternate copolymer of norbornene with isoprene catalyzed by bis(phenoxy–imine) titanium complex†
Received
14th December 2015
, Accepted 8th February 2016
First published on 9th February 2016
Abstract
A novel highly transparent alternate copolymer of norbornene with isoprene was prepared using a coordination catalytic system composed of bis(phenoxy–imine) titanium complexes and triisobutylaluminum. Distortionless enhancement by polarization transfer 13C NMR microstructure analysis of the copolymer reveals the vinyl-type addition nature of norbornene in the copolymer. The content of norbornene in the copolymer stayed constant at approximately 53%, varying the monomer feed ratio and conversion, which implied an alternating nature in the copolymer backbone. The copolymer obtained with a relatively high molecular weight (Mn = 1.05 to 2.27 × 105 g mol−1) shows good thermal stability and a moderate glass transition temperature (Tg = 75–79.5 °C). The copolymer also exhibits admirable transparency in the visible wavelengths (maximal transmittance ≈ 98%).
Introduction
As one of the most reactive strained cyclic olefins, bicyclo[2.2.1]hept-2-ene (norbornene, NBE) can be polymerized by cationic polymerization,1 ring-opening metathesis polymerization (ROMP)2 and vinyl-type addition polymerization.3 Vinyl-type polynorbornene which consists of linked strained rings has many attractive properties such as chemical resistance, high optical transparency, low birefringence and moisture absorption.4 The high rigidity of the main chain causes polynorbornene to possess a high glass transition temperature which is beyond its degradation temperature, leading to hard and brittle properties. As an efficient solution, olefin can be introduced as a comonomer to synthesize a serial of copolymers. Many transition metal catalysts can enable the copolymerization of norbornene with an α-olefin, such as ethylene,5 propylene,6 1-octene7 and styrene,8 as well as NBE derivatives.9 Regarding conjugated dienes, only a few results have been reported. Yasuda10 found NBE could be copolymerized with isoprene (IP) or 1,3-butadiene (BD) by a series of nickel compounds combined with MMAO. However, the molecular weights (Mn) of these copolymers were less than 0.9 × 104 g mol−1, and the incorporation of conjugated dienes was low. A cation scandium allyl complex and [PhNMe2H][B(C6F5)4] catalyst system was applied by Hou to synthesise a copolymer of NBE and IP with a yield lower than 50%.11 Recently, Li and co-workers12 described the random copolymerization of NBE with IP by a monotropidinyl scandium dialkyl complex/[Ph3C][B(C6F5)4]/AliBu3 ternary system and the NBE incorporation was up to 43% with a monomer feed ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (IP/NB). In our previous work, the catalytic activity of a traditional Ziegler–Natta system composed of TiCl4 and AliBu3 for the copolymerization of NBE with IP was investigated,13 and the NBE content in the copolymer could be controlled in the range of 26–60%. Nevertheless, the copolymerization of NBE with conjugated dienes still attracts interest in academic research as well as commercial applications for the potential fantastic properties. NBE units in the copolymer chain may improve its thermostability and tensile strength, while conjugated diene units can offer good elasticity, favorable solubility and a low Tg of the copolymer for better processability. The existence of –C
20 (IP/NB). In our previous work, the catalytic activity of a traditional Ziegler–Natta system composed of TiCl4 and AliBu3 for the copolymerization of NBE with IP was investigated,13 and the NBE content in the copolymer could be controlled in the range of 26–60%. Nevertheless, the copolymerization of NBE with conjugated dienes still attracts interest in academic research as well as commercial applications for the potential fantastic properties. NBE units in the copolymer chain may improve its thermostability and tensile strength, while conjugated diene units can offer good elasticity, favorable solubility and a low Tg of the copolymer for better processability. The existence of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– bonds in the copolymer can provide reaction sites for the synthesis of saturated polymers, graft polymers and functionalized polyolefins. For example, hydrogenation of the copolymer may be a new method to get saturated cyclic olefin copolymers (COC).
C– bonds in the copolymer can provide reaction sites for the synthesis of saturated polymers, graft polymers and functionalized polyolefins. For example, hydrogenation of the copolymer may be a new method to get saturated cyclic olefin copolymers (COC).
In recent years, although the majority of commercial polyolefins have been obtained by economical heterogeneous and multi-sited Ziegler–Natta catalysts, more and more single-site catalysts have become prevalent in academic research and industrial production due to their abilities in obtaining homogeneous systems and well-defined products.14 As typical single-site catalysts, bis(phenoxy–imine) early transition metal complexes occupy an important position in the polynorbornene field.15 Fujita16 synthesized a bis(phenoxy–imine) titanium complex ([2-(Ph-NCH)-3-tBuC6H3O]2TiCl2) and achieved the copolymerization of norbornene and ethylene with the disadvantage of low norbornene content. Zhang’s group17 obtained polynorbornene catalyzed by tridentatephenoxy-imine ligand [N,N,O] nickel(II) and palladium(II) complexes with MAO as cocatalyst. Tang18 successfully synthesized a family of [O-NS]TiCl3 complexes containing phenoxyimine ligands with appended alkylthio groups in the application of the copolymerization of norbornene with ethylene. These complexes exhibited many unique features, including high activity in olefin polymerizations, MAO- and borate-free polymerization catalysis and high functional group tolerance.19
Here we report the copolymerization of norbornene with isoprene obtained by a convenient catalytic system composed of bis[N,N-(3,5-di-tert-butylsalicylidene)anilinato]-titanium(IV) dichloride and triisobutyl aluminum in toluene. Different from other products,10–13 the copolymer consists of approximately 53% norbornene units in spite of different monomer feed ratios and conversion, which implied the alternate nature of the backbone. As a result, the copolymer shows an equilibrium performance, such as high molecular weight, high light transmittance, moderate glass transition temperature and good solubility in organic solvents, leading to broad application potential in optical and other fields.
Experimental
Materials
Bis(phenoxy–imine) titanium complexes were prepared as described in the literature.20 TiCl4 was purchased from Shanghai chemical reagent company. Triisobutyl aluminum (TIBA) and methylaluminoxane (MAO, 10% wt% solution in toluene) were purchased from Akzo Nobel Chemical. Norbornene (99%, Arcos) and isoprene (Sinopec Shanghai petrochemical company limited) were stirred over CaH2 for 24 h and distilled under argon before use. Toluene and n-hexane were dried over sodium/potassium and distilled under argon prior to use. The other reagents were used as received.
Polymerization
All manipulations of air- and moisture-sensitive compounds were carried out using standard Schlenk techniques under an argon atmosphere. The polymerization was carried out in a 30 mL Schlenk flask, which was repeatedly evacuated and refilled with argon. MAO, TIBA, IP and toluene solutions of NBE were added into the flask via syringe. The polymerization was initiated by the addition of a toluene solution of the titanium complex into the flask and quenched with ethanol containing 5% (vol%) hydrochloric acid. The resulting copolymer was recovered by filtration, washed with acetone, and dried in vacuum at room temperature for 24 h.
Preparation of copolymer film
200 mg of the copolymer was dissolved in 10 mL of tetrahydrofuran at room temperature and poured onto a smooth glass plate. After 3 days, the plate was placed under vacuum until it was of constant weight. The film was stripped from the plate and used for optical testing.
Measurements and characterizations
The NMR data were recorded on a Bruker Avance DMX500 spectrometer operated at 500 MHz for 1H NMR and 126 MHz for 13C NMR using CDCl3 as the solvent at ambient temperature with TMS as internal reference. The distortionless enhancement by polarization transfer (DEPT) 135 spectrum was achieved by a Bruker Avance DMX500 spectrometer at 126 MHz for 13C NMR. The number-average molecular weight (Mn) and the polydispersity index (PDI) of the polymer samples were determined by a gel permeation chromatography (GPC, Waters 1525/2414) system operated at 40 °C with THF as eluent at a flow rate of 1.0 mL min−1 against the standard polystyrene samples. The glass transition temperatures (Tg) were determined by differential scanning calorimetry (DSC, DSC TA Q100) at heating and cooling rates of 10 °C min−1 under a nitrogen atmosphere. The thermal gravimetric analysis (TGA) measurements were performed on a Perkin-Elmer instrument TGA 7 from room temperature to 500 °C at a rate of 20 °C min−1 under a dry nitrogen atmosphere. Light transmittance of the copolymer thin film in the range of visible wavelengths was measured by a CARY 100 BIO UV/vis spectrometer.
 |
| | Fig. 1 Linear fitting of the NBE/IP copolymerization system catalyzed by 1/TIBA according to Kelen–Tϋdόs method. | |
Results and discussion
Copolymerization of NBE with IP
The copolymerization of NBE with IP was carried out using bis(phenoxy–imine) titanium complexes (Scheme 1) activated by MAO or TIBA, the results are compiled in Table 1.
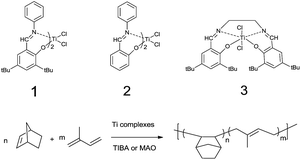 |
| | Scheme 1 Structure of bis(phenoxy–imine) titanium complexes and the scheme of copolymerization. | |
Table 1 Copolymerization of NBE with IP by different catalytic systemsa
| Entry |
Catalyst system |
Al/Ti (mol/mol) |
Activity (g molTi−1 h−1) |
Mnb (105) |
PDIb |
[Ti] = 4.2 × 10−5 mol, [NBE + IP]/[Ti] = 200 (molar ratio), [NBE]/[IP] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (molar ratio), polymerized at 40 °C in toluene. Determined by GPC in THF against polystyrene standard. The fraction dissolved in THF. 1 (molar ratio), polymerized at 40 °C in toluene. Determined by GPC in THF against polystyrene standard. The fraction dissolved in THF. |
| 1 |
1/TIBA |
5 |
1680 |
1.52 |
3.32 |
| 2 |
1/MAO |
300 |
1900 |
0.21c |
1.86c |
| 3 |
2/TIBA |
5 |
— |
— |
— |
| 4 |
2/MAO |
300 |
— |
— |
— |
| 5 |
3/TIBA |
5 |
— |
— |
— |
| 6 |
3/MAO |
300 |
— |
|
|
The data in Table 1 indicates that when either activated by TIBA (Entry 1) or MAO (Entry 2), complex 1 was active toward the copolymerization of NBE with IP. In contrast to the product prepared from the 1/MAO system (Entry 2), the copolymer obtained from the 1/TIBA system (Entry 1) showed higher molecular weight (Mn = 1.52 × 105 g mol−1) with good solubility in normal organic solvents. Though the narrower molecular weight distribution of the copolymer (Entry 2) was also attractive when MAO was used as activator, the copolymer might be cross-linked as only part of it dissolved in organic solvents. However, it was proved that neither complex 2 nor complex 3 showed catalytic activity towards the copolymerization of NBE with IP. Compared to complex 1, the lack of two tert-butyls in complex 2 reduced the electron density in the centre metal, leading to lower activity in copolymerization. When referring to complex 3, the ethylenediamine increased the steric hindrance around the metal centre, which made the norbornene monomer difficult to insert. Therefore, the complex 1/TIBA binary system was chosen to explore the effect of different reactive conditions on the copolymerization (Table 2).
Table 2 Copolymerization of NBE with IP catalyzed by the 1/TIBA binary systema
| Entry |
FNBE/FIP (mol/mol) |
Al/Ti (mol/mol) |
Time (min) |
Activity (g molTi−1 h−1) |
Mnb (105) |
PDIb |
fNBEc (mol%) |
| [Ti] = 4.2 × 10−5 mol, [NB + IP]/[Ti] = 200 (molar ratio), polymerized at 40 °C in toluene. Determined by gel permeation chromatography in tetrahydrofuran against polystyrene standard. fNBE is the mol fractions of the norbornene in the copolymer. Determined by 1H NMR spectroscopy in CDCl3. Polymerized at 60 °C. Not determined. |
| 1 |
5/5 |
5 |
360 |
2442 |
1.78 |
2.54 |
53 |
| 2 |
5/5 |
10 |
360 |
748 |
1.73 |
2.41 |
54 |
| 3d |
5/5 |
5 |
360 |
2093 |
1.29 |
2.51 |
57 |
| 4 |
5/5 |
5 |
10 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 429 429 |
1.77 |
2.56 |
54 |
| 5 |
5/5 |
5 |
30 |
8476 |
2.57 |
2.71 |
55 |
| 6 |
5/5 |
5 |
120 |
5048 |
1.46 |
3.38 |
47 |
| 7 |
5/5 |
5 |
180 |
4778 |
1.53 |
2.66 |
53 |
| 8 |
5/5 |
5 |
240 |
3587 |
1.81 |
2.44 |
55 |
| 9 |
10/0 |
5 |
360 |
190 |
—e |
— |
100 |
| 10 |
0/10 |
5 |
360 |
110 |
— |
— |
0 |
| 11 |
3/7 |
5 |
360 |
1885 |
2.27 |
3.58 |
47 |
| 12 |
7/3 |
5 |
360 |
285 |
1.05 |
2.83 |
56 |
| 13 |
3/7 |
5 |
10 |
6142 |
1.85 |
2.33 |
55 |
| 14 |
3/7 |
5 |
20 |
8357 |
2.63 |
2.31 |
53 |
| 15 |
3/7 |
5 |
40 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 964 964 |
2.38 |
2.94 |
52 |
The Ti complex 1/TIBA binary system displayed high activity towards the copolymerization of NBE with IP, in spite of the very low activity in the homopolymerization of the two monomers respectively. A higher TIBA amount (Al/Ti = 10, Entry 2) led to lower catalytic activity due to an enhancement in the chain transfer reaction. When the polymerization temperature increased from 40 to 60 °C (Entry 1 and 3), the catalyst activity decreased by 350 g molTi−1 h−1 and a part of the catalyst may decompose at high temperature. The Mn and catalytic activity decreased with increasing NBE feed ratio, while the NBE content of the copolymer remained at a certain value (nearly 53%). The reactivity ratios of NBE and IP in the copolymerization catalyzed by 1/TIBA system were estimated using the Kelen–Tϋdόs method (Fig. 1).21 A series of copolymers with low monomer conversion (<15%) were obtained by varying the monomer feed ratio and the reaction time, and the reactivity ratios were calculated as rNBE = 0.30 and rIP = 0.01 for NBE and IP, respectively. The relationship between polymerization time and monomer conversion was investigated, what’s more the content of NBE in the copolymer was determined by 1H NMR with monomer feed ratios of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 3
5 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (NBE/IP). As shown in Fig. 2, with increasing polymerization time, the monomer conversion exhibited a rapid increase in the primary stage, while the polymerization rate slowed down sharply after 2 hours with the conversion at approximately 80% and 65%, respectively. Furthermore, the NBE content in the resulting copolymer always stayed at a constant level, regardless of the polymerization time. To summarize the polymerization results, monomer feed ratio and conversion both made no difference in the composition of the copolymers, which further illustrated the high probability of alternation in chain propagation.
7 (NBE/IP). As shown in Fig. 2, with increasing polymerization time, the monomer conversion exhibited a rapid increase in the primary stage, while the polymerization rate slowed down sharply after 2 hours with the conversion at approximately 80% and 65%, respectively. Furthermore, the NBE content in the resulting copolymer always stayed at a constant level, regardless of the polymerization time. To summarize the polymerization results, monomer feed ratio and conversion both made no difference in the composition of the copolymers, which further illustrated the high probability of alternation in chain propagation.
 |
| | Fig. 2 The relationship between polymerization time and monomer conversion as well as NBE content in copolymers with different feed ratios. | |
The microstructure of the copolymers was determined by 1H and 13C NMR analyses. The 1H NMR spectrum of PIP is shown in Fig. 3a. The signals attributed to the allylic protons of the 3,4- and 1,4-units in PIP were observed at 4.7 and 5.2 ppm, respectively. As shown in the 1H NMR spectrum of the copolymer (Fig. 3b), the absence of a signal at 4.7 ppm means there are no 3,4-units of PIP in the copolymer due to insufficient space around the active center in chain propagation. The signal at 5.2 ppm is attributed to the presence of allylic protons. Therefore, the copolymer was further characterized by 13C NMR (Fig. 3c), and the signals appearing in the ranges of 23–24 ppm (C10), 27–33 ppm (C5–8,12), and 40–47 ppm (C1–4) were attributed to the alkyl carbons of the PNBE and PIP segments in the copolymer, respectively. The signals appearing in the range of 120–130 ppm (C9,11) correspond to the olefin carbons. Because of the existence of a double bond in the IP units, it was difficult to infer the structure of the NBE units in the copolymer. From this point of view, DEPT 135 13C NMR was used to identify the accurate microstructure of the copolymer.21 As shown in Fig. 3d, the negative signal (–CH2–) assignable to the bridge carbon of the ROMP NBE unit (C7′) was not observed in the range of 40 to 45 ppm, indicating that NBE copolymerized with IP via vinyl-type polymerization.
 |
| | Fig. 3 1H and 13C NMR spectra of PIP and the copolymer: (a) 1H NMR spectrum of PIP and (b) 1H NMR spectrum of copolymer (Table 2, Entry 1). (c) 13C NMR spectrum of copolymer (Table 2, Entry 1) and (d) DEPT 135 13C NMR spectrum of copolymer (Table 2, Entry 1). | |
The copolymer molecular weight had nothing to do with the polymerization time, as well as the molecular weight distribution. This revealed that the polymerization experienced an extremely rapid initiation stage. The experimental data obtained at present was not sufficient to infer the exact copolymer mechanism. A plausible mechanism for the alternate copolymerization is provided in Fig. S1.†
Thermal properties of the copolymers
The glass transition temperature (Tg) of the copolymers with different NBE feed ratios determined by DSC were 75 °C, 76 °C, and 79.5 °C for Entries 9, 1 and 10 in Table 2 (Fig. 4), respectively, with contents of NBE in the copolymer increasing from 47 to 56%. The DSC curves showed only one glass transition temperature in the range from 70 °C to 80 °C which proved the successful synthesis of copolymer. Further, the lack of melting or cooling peaks in the DSC curves also indicated the absence of homopolymers. TGA was adopted to examine the thermal stability of the copolymer. The curves showed that these three samples underwent almost the same decomposition process. Thermal gravimetric analysis measurements indicate that the copolymer began to decompose at nearly 400 °C (Fig. 5), which was a relatively high decomposition temperature compared with other diolefin copolymers. The small decrease of mass in the temperature range from 100 °C to 150 °C was caused by the volatilization of toluene which was inside the copolymer. Even though there were many double bonds in the backbone, the alternate structure enhanced the thermal stability of the copolymer remarkably.
 |
| | Fig. 4 DSC curves of the copolymers (Table 2): 1-Entry 9; 2-Entry 1; 3-Entry 10. | |
 |
| | Fig. 5 TGA curves of the copolymers (Table 2): 1-Entry 11; 2-Entry 1; 3-Entry 12. | |
Transparency of the copolymer
It is known that the insertion of norbornene units can improve the transparency of a copolymer significantly. The copolymer of norbornene and ethylene was successfully commercialized as an optical device due to its excellent transparency in the 1980s. The copolymer obtained here exhibited an excellent transparency characterized by a UV/vis spectrometer with a thin film sample (Table 2, Entry 1). As shown in Fig. 6, the sample showed high light transmittance over 90% in the visible light wavelengths (380–780 nm). Compared with the commercial copolymer, the replacement of the comonomer from ethylene to isoprene has no significant effect on the transparency of the copolymer, but improves the processability. The high light transmittance in the visible wavelengths indicated that there was no phase separation occurring in the process of casting the film. Further, this could be evidence for the successful synthesis of the copolymer and revealed the random structure of the copolymer backbone.
 |
| | Fig. 6 The light transmittance of the copolymer obtained (Table 2, Entry 1). | |
Conclusions
Copolymers of NBE with IP were successfully prepared using a bis[N,N-(3,5-di-tert-butylsalicylidene)anilinato]-titanium(IV) dichloride and triisobutyl aluminum binary catalytic system in toluene. The NBE content in the copolymer was close to 53%, in spite of the monomer feed ratio and conversion. It can be inferred that the copolymer possessed an approximate alternate backbone. The DEPT 135 13C NMR analysis confirmed that NBE was polymerized through vinyl-addition. The insertion of IP improved the solubility and processability significantly, resulting in a moderate glass transition temperature. With the wavelength increase in the range of visible light, the copolymer showed an excellent light transmittance from 90% to 98%.
Acknowledgements
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (21174121), the Special Funds for Major Basic Research Projects (G2011CB606001).
Notes and references
-
(a) J. P. Kennedy and H. S. Makowski, J. Macromol. Sci., Chem., 1967, A1, 345–370 CrossRef;
(b) G. Myagmarsuren, K. S. Lee, O. Y. Jeong and S. K. Ihm, Catal. Commun., 2003, 4, 615–619 CrossRef CAS.
-
(a) A. W. Andersen and N. G. Merkling, Polymeric Bicyclo-(2.2.1)-2-heptene, U.S. Pat., 2, 721,189, August 30, 1954;
(b) Z. Li, J. Ma, C. Cheng, K. Zhang and K. L. Wooley, Macromolecules, 2010, 43, 1182–1184 CrossRef CAS;
(c) X. Li, X. F. Ni and Z. Q. Shen, Acta Polym. Sin., 2012, 11, 1270–1275 CrossRef;
(d) M. Al-Hashimi, M. D. Abu Bakar, K. Elsaid, D. E. Bergbreiterc and H. S. Bazzi, RSC Adv., 2014, 4, 43766–43771 RSC.
-
(a) Q. Wu and Y. Y. Lu, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 1421–1425 CrossRef CAS;
(b) F. Blank and C. Janiak, Coord. Chem. Rev., 2009, 253, 827–861 CrossRef CAS;
(c) K. Nomura, S. Patamma, H. Matsuda, S. Katao, K. Tsutsumia and H. Fukuda, RSC Adv., 2015, 5, 64503–64513 RSC.
-
(a) C. Janiak and P. G. Lassahn, Macromol. Rapid Commun., 2001, 22, 479–492 CrossRef CAS;
(b) T. F. A. Haselwander, W. Heitz, S. A. Krϋgel and J. H. Wendorff, Macromolecules, 1997, 30, 5345–5351 CrossRef CAS;
(c) K. L. Makovetskii, Polym. Sci., Ser. C, 2008, 50, 22–38 CrossRef;
(d) B. L. Good, Late transition metal polymerization catalysis, ed. B. Rieger, B. L. Saunders, S. Kacker and S. Striegler, Wiley-VCH, Weinheim, Germany, 2003, pp. 101–154 Search PubMed.
-
(a) J. Y. Liu, P. Tao, Y. X. Wang and Y. S. Li, RSC Adv., 2014, 4, 19433–19439 RSC;
(b) W. Apisuk, A. G. Trambitas, B. Kitiyanan, M. Tamm and K. Nomura, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 2575–2580 CrossRef CAS.
-
(a) R. Tanaka, Y. Nakayama and T. Shiono, Polym. Chem., 2013, 4, 3974–3980 RSC;
(b) V. N. Dougnac, B. C. Peoples, F. M. Rabagliati, G. B. Galland and R. Quijada, Polym. Bull., 2012, 69, 925–935 CrossRef CAS.
-
(a) Z. G. Cai, R. Harada, Y. Nakayama and T. Shiono, Macromolecules, 2010, 43, 4527–4531 CrossRef CAS;
(b) Y. M. Liu, O. Y. Meng, X. H. He, Y. W. Chen and K. T. Wang, J. Appl. Polym. Sci., 2013, 128, 216–223 CrossRef.
-
(a) H. T. Ban, H. Hagihara, Y. Tsunogae, Z. G. Cai and T. Shiono, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 65–71 CrossRef CAS;
(b) F. Qian, D. J. Chen, D. Y. Feng, L. B. Jiao, Z. G. Peng and L. X. Pei, J. Polym. Res., 2014, 21, 497 CrossRef.
-
(a) S. Kaita, K. Matsushita, M. Tobita, Y. Maruyama and Y. Wakatsuki, Macromol. Rapid Commun., 2006, 27, 1752–1756 CrossRef CAS;
(b) K. Ogata, A. Watanabe, T. Yunokuchi and A. Toyota, Inorg. Chem. Commun., 2008, 11, 215–219 CrossRef CAS;
(c) J. W. Tian, X. H. He, J. Y. Liu, X. D. Denga and D. F. Chen, RSC Adv., 2015, 5, 61851–61860 RSC.
- H. Suzuki, S. Matsumura, Y. Satoh, K. Sogoh and H. Yasuda, React. Funct. Polym., 2004, 59, 253–266 CrossRef CAS.
- N. Yu, M. Nishiura, X. F. Li, Z. F. Xi and Z. M. Hou, Chem.–Asian. J., 2008, 3, 1406–1414 CrossRef CAS PubMed.
- S. Q. Liu, G. X. Du, J. Y. Long, S. W. Zhang and X. F. Li, Macromolecules, 2014, 47, 3567–3573 CrossRef CAS.
- X. Li, X. F. Ni and Z. Q. Shen, Chem. J. Chin. Univ., 2012, 5, 1095–1099 Search PubMed.
-
(a) X. F. Li and Z. M. Hou, Coord. Chem. Rev., 2008, 252, 1842–1869 CrossRef CAS;
(b) X. F. Ni, W. W. Zhu and Z. Q. Shen, Polymer, 2010, 51, 2548–2555 CrossRef CAS.
-
(a) D. Q. Song, H. L. Mu, X. C. Shi, Y. G. Li and Y. S. Li, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 562–570 CrossRef CAS;
(b) K. T. Wang, Y. W. Chen, X. H. He, Y. M. Liu and W. H. Zhou, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 3304–3313 CrossRef CAS.
- Y. Yoshida, J. Mohri, S. Ishii, M. Mitani, J. Saito, S. Matsui, H. Makio, T. Nakano, H. Tanaka, M. Onda, Y. Yamamoto, A. Mizuno and T. Fujita, J. Am. Chem. Soc., 2004, 126, 12023–12032 CrossRef CAS PubMed.
- A. K. Li, J. X. Chen and Z. C. Zhang, J. Appl. Polym. Sci., 2009, 113, 1642–1650 CrossRef CAS.
- X. H. Yang, Z. Wang, X. L. Sun and Y. Tang, Dalton Trans., 2009, 8945–8954 RSC.
-
(a) T. Matsugi and T. Fujita, Chem. Soc. Rev., 2008, 37, 1264–1277 RSC;
(b) M. Mitani, J. Saito, S. Ishii, Y. Nakayama, H. Makio, N. Matsukawa, S. Matsui, J. Mohri, R. Furuyama, H. Terao, H. Bando, H. Tanaka and T. Fujita, Chem. Rec., 2004, 4, 137–158 CrossRef CAS PubMed.
- J. Saito, M. Mitani, S. Matsui, Y. Tohi, H. Makio, T. Nakano, H. Tanaka, N. Kashiwa and T. Fujita, Macromol. Chem. Phys., 2002, 203, 59–65 CrossRef CAS.
-
(a) K. J. Ivin, D. T. Laverty and J. J. Rooney, Macromol. Chem., 1977, 178, 1545–1560 CrossRef CAS;
(b) M. Arndt, R. Engehausen, W. Kaminsky and K. Zoumis, J. Mol. Catal. A: Chem., 1995, 101, 171–178 CrossRef CAS;
(c) T. Hasan, T. Ikeda and T. Shiono, Macromolecules, 2004, 37, 7432–7436 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26641f |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (IP/NB). In our previous work, the catalytic activity of a traditional Ziegler–Natta system composed of TiCl4 and AliBu3 for the copolymerization of NBE with IP was investigated,13 and the NBE content in the copolymer could be controlled in the range of 26–60%. Nevertheless, the copolymerization of NBE with conjugated dienes still attracts interest in academic research as well as commercial applications for the potential fantastic properties. NBE units in the copolymer chain may improve its thermostability and tensile strength, while conjugated diene units can offer good elasticity, favorable solubility and a low Tg of the copolymer for better processability. The existence of –C
20 (IP/NB). In our previous work, the catalytic activity of a traditional Ziegler–Natta system composed of TiCl4 and AliBu3 for the copolymerization of NBE with IP was investigated,13 and the NBE content in the copolymer could be controlled in the range of 26–60%. Nevertheless, the copolymerization of NBE with conjugated dienes still attracts interest in academic research as well as commercial applications for the potential fantastic properties. NBE units in the copolymer chain may improve its thermostability and tensile strength, while conjugated diene units can offer good elasticity, favorable solubility and a low Tg of the copolymer for better processability. The existence of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– bonds in the copolymer can provide reaction sites for the synthesis of saturated polymers, graft polymers and functionalized polyolefins. For example, hydrogenation of the copolymer may be a new method to get saturated cyclic olefin copolymers (COC).
C– bonds in the copolymer can provide reaction sites for the synthesis of saturated polymers, graft polymers and functionalized polyolefins. For example, hydrogenation of the copolymer may be a new method to get saturated cyclic olefin copolymers (COC).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (molar ratio), polymerized at 40 °C in toluene.b Determined by GPC in THF against polystyrene standard.c The fraction dissolved in THF.
1 (molar ratio), polymerized at 40 °C in toluene.b Determined by GPC in THF against polystyrene standard.c The fraction dissolved in THF.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 429
429![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 964
964![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 3
5 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (NBE/IP). As shown in Fig. 2, with increasing polymerization time, the monomer conversion exhibited a rapid increase in the primary stage, while the polymerization rate slowed down sharply after 2 hours with the conversion at approximately 80% and 65%, respectively. Furthermore, the NBE content in the resulting copolymer always stayed at a constant level, regardless of the polymerization time. To summarize the polymerization results, monomer feed ratio and conversion both made no difference in the composition of the copolymers, which further illustrated the high probability of alternation in chain propagation.
7 (NBE/IP). As shown in Fig. 2, with increasing polymerization time, the monomer conversion exhibited a rapid increase in the primary stage, while the polymerization rate slowed down sharply after 2 hours with the conversion at approximately 80% and 65%, respectively. Furthermore, the NBE content in the resulting copolymer always stayed at a constant level, regardless of the polymerization time. To summarize the polymerization results, monomer feed ratio and conversion both made no difference in the composition of the copolymers, which further illustrated the high probability of alternation in chain propagation.





