Hydrophilic sulfonic acid-functionalized micro-bead silica for dehydration of sorbitol to isosorbide†
Jun Shi,
Yuhua Shan*,
Yuan Tian,
Yu Wan,
Yitian Zheng and
Yangyang Feng
Advanced Catalysis and Green Manufacturing Collaborative Innovation Center, Changzhou University, Changzhou 213164, China. E-mail: yhshan@cczu.edu.cn
First published on 19th January 2016
Abstract
Different (3-mercaptopropyl)trimethoxysilane (MPTS) loadings of sulfonic acid-functionalized micro-bead silica (SA-SiO2) were prepared by silylation and oxidation, and characterized by elemental analysis, SEM, FT-IR, TGA, NH3-TPD, BET N2 adsorption–desorption and 13C NMR CP/MAS. The as-prepared SA-SiO2 showed strong hydrophilic nature and excellent catalytic performance for dehydration of sorbitol to isosorbide. The selectivity to isosorbide is obviously affected by the MPTS loading. Using SA-SiO2 as a solid catalyst with 60.5% MPTS loading, 100% sorbitol conversion and 84% yield of isosorbide are achieved at 120 °C for 10 h under vacuum. The catalyst was reused 10 times without noticeable loss of activity and selectivity.
Introduction
With environmental concerns, use of green and renewable raw materials to produce chemicals has become the trend of the times. Therefore, the interest in biomass conversion into chemicals has increased rapidly during the last few years.1–6 Today, sorbitol, which can be conveniently obtained from cellulose (considered the most abundant and cheap carbon source)7–9 via glucose, is one of the “top ten” platform chemicals in biorefining according to the US Department of Energy,10–14 and its most popular dehydrated product is isosorbide15 – an important platform chemical for the replacement of traditional oil resource products in the future.16–20The dehydration of sorbitol to isosorbide (Scheme 1) is conventionally achieved using a liquid acid like sulfuric acid, hydrochloric acid or p-toluenesulfonic acid as a homogeneous catalyst in industrial production, which provides a yield of isosorbide (70–77%) at 130 °C under high vacuum conditions within a few hours.21 But use of homogeneous catalysts introduces many troubles such as difficulties in product separation, a corrosive hazard for the reactors and catalyst regeneration from the reaction system.20 Without adding any acid catalyst, in the dehydration of sorbitol in high temperature water (250–300 °C) for 1 h, 57% yield of isosorbide was obtained, reported by Yamaguchi et al.20,22 In addition, dehydration of pure sorbitol under microwaves at 160 °C showed sorbitol conversion and isosorbide selectivity of 100% and 60%,23 respectively.
Therefore, the research of sorbitol dehydration has been focused on developing solid acid catalysts.17,24–27 Many solid catalysts have been reported with different yields of isosorbide at suitable temperatures, such as sulfated copper oxides (67.3%, 200 °C),17 sulfated zirconia (61%, 210 °C),24 sulfated tin oxide (65%, 180 °C),25 metal phosphate (70.3%, 250 °C),28 molten salt hydrate medium (85%, 200 °C),27,29,30 and Amberlyst-15 resin (71.8%, 250 °C).31
However, most of these catalysts require comparatively high reaction temperature (200–300 °C) and the yield of isosorbide is not high. Therefore, the development of an efficient solid acid catalyst which catalyzes this reaction at moderate reaction conditions and leads to a high yield of isosorbide is highly desirable.
Polymer-supported Brønsted acid catalysts SO3H–PS–SO3H32 have been used for dehydration of sorbitol at 150 °C, with a maximum yield of 1,4-anhydro-D-sorbitol of 90% within 4 h. However, the yield of isosorbide is not high. Alternatively, a superhydrophobic mesoporous acid catalyst named as P–SO3H has been synthesized and used for dehydration of sorbitol to isosorbide,33 with a maximum yield (87%) of isosorbide at 140 °C within 10 h. Although these hydrophobic polymer-supported sulfonic acid catalysts show high yield, they are deactivated quickly because of their surface affinity to humins and cokings.
Silica sulfuric acid catalysts have been widely studied for a great number of acid-catalyzed organic reactions due to their heterogeneous nature, cheapness and availability.34 A simple in situ method to prepare water-stable acidic mesoporous sulfonated silica without any surfactant or template was reported by Hasan,35 and it has good catalytic performance for hydrolysis and alkylation reactions. Micro-bead silica has the advantages of hydrophilicity, high BET surface area and special porous structure.36,37 And the chemical bond linking method has good prospects for industrial application since it can fix organic groups on a carrier surface firmly.38,39 In this work, we report a simple and low-cost synthesis of a hydrophilic sulfonic acid-functionalized micro-bead silica catalyst named SA-SiO2 and evaluated its catalytic performance in dehydration of sorbitol to isosorbide in solvent-free condition. As we expected, SA-SiO2 gives good sorbitol conversion and isosorbide selectivity, and has excellent recyclability, compared with other reported catalysts.
Experimental details
Materials
Hβ (Si/Al = 25), HZSM-5 (Si/Al = 40) and MCM-49 (Si/Al = 30) were bought from Chinese NanKai University. Micro-bead silica (280–600 μm) was purchased from Qingdao Haiyang Chemical Co. Ltd. (3-Mercaptopropyl)trimethoxysilane (MPTS) was purchased from Shanghai Ziyi-reagent Chemical Co. Ltd. Amberlyst-15 resin was purchased from Shanghai Host Chemical Co. Ltd. Calcium hydride (CaH2), hydrogen peroxide (30% H2O2), hydrochloric acid (37% HCl), sulfuric acid (98% H2SO4), acetic acid, acetonitrile, toluene, glycol dimethyl ether, tergitol(tm)xh-(nonionic) (P123) and tetraethyl orthosilicate (TEOS) were bought from SCRC (Sinopharm Chemical Reagent Co. Ltd, Shanghai).Catalyst preparation
By adjusting the ratio of MPTS to micro-bead silica, different SA-SiO2-X samples were obtained. For example, a catalyst loading of 60.5% MPTS is represented as SA-SiO2-60.5. The elemental analysis of the SA-SiO2 catalysts is shown in Table 1S.†
Catalyst characterization
NMR spectra were collected by using a Bruker instrument (Avance III 500 MHz). The Brunauer–Emmett–Teller (BET) surface areas of catalysts were calculated according to the nitrogen adsorption–desorption isotherms method using Micromeritics ASAP2010C equipment. Before this analysis,41 the samples were degassed at 150 °C for at least 10 h to remove moisture and volatile impurities. Pore size distribution and pore volume were determined by the density functional theory method. FT-IR spectra were obtained using a Bruker FT-IR spectrometer (TENSOR27) with a high-temperature vacuum chamber.42 Thermogravimetric analysis (TGA) measurements were carried out by an SDT Q600 instrument in a nitrogen atmosphere and the constant heating rate was 10 °C min−1. NH3-TPD was carried out using a BE-CAT-B-82 instrument equipped with a thermal conductivity detector. The scanning electron microscopy (SEM) experiments were conducted with a scanning electron microscope (SUPRA 55 SAPPHIRE). The compositions of the catalysts were analyzed by inductively coupled plasma analysis (ICP) using a PerkinElmer 2400 Series II CHNS device. The surface hydrophilicity of catalyst was measured by liquid contact angle measurements using a drop shape analyzer (DSA100, KRÜSS GmbH). The operation was carried out according to the following procedure: SA-SiO2 powders were pressed into a sheet and then the sheet and sorbitol/isosorbide were placed in a 100 °C constant temperature box for 1 h. The molten sorbitol/isosorbide was dropped onto the SA-SiO2 sheet surface rapidly and a picture taken quickly. A diagram of the apparatus is shown in Fig. S6.†The acidity of the SA-SiO2 catalysts was determined by acid–base titration in an ultrasonic pool, according to the following definition:
| Total acidity = (V1 − V0) × CNaOH/W |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Concentration of standard NaOH solution (mol L−1), W: weight of test sample (g).
Concentration of standard NaOH solution (mol L−1), W: weight of test sample (g).
Catalyst performance evaluation and product analysis
The catalytic reaction was carried out in a 250 mL round-bottom flask equipped with a vacuum pump (in order to maintain the reaction pressure below 1000 Pa) with a magnetic stirrer (300 rpm.). Sorbitol (50 g) was added in a three-neck flask, followed by heating at 100 °C to yield a clear melt. Then, the catalyst was added and the reactor was heated to reaction temperature with an oil bath pot. During the heating reaction, water was continuously removed under reduced pressure to promote the reaction equilibrium shift to the positive direction.Products were analyzed by HPLC (Agilent LC1260) equipped with a Zorbax NH2 column (4.6 × 200 mm, 5 μm particle size) and an evaporative light-scattering detector. The sugar products were separated by reversed-phase mode. The mobile phase was acetonitrile–water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) with a flow rate of 0.5 mL min−1. The column was thermostatically controlled at room temperature. The selectivity or yield of sorbitan or isosorbide was based on molar composition. The isolated yield is calculated by the following definition:
20) with a flow rate of 0.5 mL min−1. The column was thermostatically controlled at room temperature. The selectivity or yield of sorbitan or isosorbide was based on molar composition. The isolated yield is calculated by the following definition:
| yieldisolated = (mol of isolated product)/(mol of sorbitol) × 100% |
Procedure for catalyst regeneration
To better test the stability of used catalyst, regeneration was performed with the following procedure: after reaction, the used catalyst was recycled by filtration, and washed in ultrasonic pool with water, glycol dimethyl ether, and water sequentially to remove the coke formed in the reaction, then dried at 100 °C for the next run.Results and discussion
Catalyst characterization
The sulfonic acid-functionalized catalyst was characterized by various physicochemical analyses. Fig. 1 shows FT-IR spectra of SA-SiO2-60.5 and SA-SiO2-60.5 after ten recycles. The oxidation product of MPTS displays characteristic bands at 1360–960 cm−1 and 800–510 cm−1. The bands at 601 cm−1 and 526 cm−1 indicate the presence of C–S bond stretching vibrations, while the absorption peak at about 1170 cm−1 is associated with a sulfonic acid group because of the presence of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond.43 The strong and broad peak at around 3440 cm−1 is assigned to the stretching vibration of –OH. Furthermore, the SA-SiO2-60.5 catalyst shows absorption peaks at 688, 601, and 526 cm−1. In addition, these absorption peaks all show red shifts due to the electron inductive effect of O
O bond.43 The strong and broad peak at around 3440 cm−1 is assigned to the stretching vibration of –OH. Furthermore, the SA-SiO2-60.5 catalyst shows absorption peaks at 688, 601, and 526 cm−1. In addition, these absorption peaks all show red shifts due to the electron inductive effect of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond.44 All the results indicate successful introduction of sulfonic acid groups in the SA-SiO2-60.5 sample.
O double bond.44 All the results indicate successful introduction of sulfonic acid groups in the SA-SiO2-60.5 sample.
The SEM images of SiO2 and SA-SiO2-60.5 are shown in Fig. 2. From the SEM images, it can be seen that the surface of SA-SiO2-60.5 catalyst is rough while that of the micro-bead silica is relatively smooth. This is caused by the sulfonic acid groups chemically bonded on the micro-bead silica.
The (CP/MAS) 13C-NMR spectra of SA-SiO2-60.5 and its precursor (unoxidized product) are shown in Fig. 3. The chemical shifts of saturated carbon are generally at 0–70, while those of the unsaturated carbon are usually at 100–165. As shown in Fig. 3A, the chemical shift peaks at 16.49 and 23.42 are associated with the saturated carbon atom attached to a silicon atom and saturated carbon atom attached to a carbon atom of both ends, respectively. The chemical shift peak at 58.95 corresponds to the saturated carbon atom attached to an oxidized sulfur atom.45 Compared with Fig. 3A, B shows the chemical shift peak at 47.61, which is associated with a saturated carbon atom attached to an unoxidized sulfur atom. Interestingly the peaks of other carbon atoms in Fig. 3B show red shifts because of the oxidation of sulfur atom. In a word, we can confirm that the product of MPTS loading is oxidized and propylsulfonic acid group is successfully connected to SiO2 by chemical bonding.
TGA of SA-SiO2-60.5 catalyst is shown in Fig. 4. Firstly, a slow weight loss in the range of 0–250 °C was noticed. Then a sharp weight loss was observed between 250 and 400 °C, which indicates desorption of the sulfonic groups on the surface of the micro-bead silica. The weight loss above 400 °C is due to the dehydration of hydroxyl groups in the pore walls of micro-bead silica.40 Thus it can be concluded that the SA-SiO2-60.5 catalyst is thermally stable and suitable for use at less than 250 °C.
The acidity, surface area, pore volume and pore size of various acid catalysts are shown in Table 1. The sulfonic resin has the maximum acidity (4530 μmol g−1) among these solid catalysts while its surface area is very poor (49.1 m2 g−1). Compared with the Amberlyst-15 resin, the SA-SiO2 has larger surface area and exhibits an appropriate high acidity and pore size. Furthermore, as shown in Table 1 (entries 1, 2, 3, 4, 5, 6, 7, 8 and 9), the acid concentration can be adjusted, and the acidity varied from 230 μmol g−1 to 1290 μmol g−1. The pore volume and pore size decreased with an increase of MPTS content. Pore size distributions of used SA-SiO2-60.5 and SBA-15-SO3H are shown in Fig. S4.†
| Entry | Catalyst | SBET (m2 g−1) | Vp (cm3 g−1) | Mean Dp (nm) | Total aciditya (μmol g−1) | Acidity densityb (μmol m−2) |
|---|---|---|---|---|---|---|
| a Determined by NH3-TPD (Fig. 3S) except SA-SiO2-60.5.b Acidity density = total acid amount/BET surface area.c Undetectable.d After ten recycles. | ||||||
| 1 | Micro-bead silica | 350.9 | 0.84 | 11.3 | —c | —c |
| 2 | SA-SiO2-10.6 | 345.0 | 0.68 | 11.1 | 230 | 0.66 |
| 3 | SA-SiO2-22.5 | 310.3 | 0.51 | 10.5 | 340 | 1.10 |
| 4 | SA-SiO2-33.1 | 273.7 | 0.43 | 9.9 | 490 | 1.79 |
| 5 | SA-SiO2-40.9 | 221.1 | 0.39 | 9.6 | 610 | 2.76 |
| 6 | SA-SiO2-52.0 | 160.5 | 0.33 | 8.1 | 710 | 4.42 |
| 7 | SA-SiO2-60.5 | 140.9 | 0.29 | 7.3 | 840 | 5.96 |
| 8 | SA-SiO2-71.9 | 109.7 | 0.17 | 6.0 | 970 | 8.84 |
| 9 | SA-SiO2-83.3 | 48.3 | 0.09 | 4.9 | 1290 | 26.71 |
| 10 | H2SO4 | —c | —c | —c | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
—c |
| 11 | Amberlyst-15 | 49.1 | 0.29 | 40.0 | 4530 | 92.26 |
| 12 | SBA-15-SO3H | 379.6 | 0.92 | 3.8 | 810 | 2.13 |
| 13 | Hβ (25) | 650.9 | 0.25 | 0.65 | 490 | 0.75 |
| 14 | HZSM-5 (40) | 451.7 | 0.14 | 0.54 | 320 | 0.71 |
| 15 | MCM-49 (30) | 457 | 0.46 | 0.71 | 435 | 0.95 |
| 16 | SA-SiO2-60.5d | 140.1 | 0.26 | 7.1 | 790 | 5.64 |
| 17 | SBA-15-SO3Hd | 303.6 | 0.69 | 3.1 | 150 | 0.49 |
Catalyst performance
The catalytic performance of SA-SiO2 catalysts for dehydration of sorbitol to isosorbide was evaluated in solvent-free and vacuum conditions, which are helpful to remove water from the reaction system to promote the reaction equilibrium shift to the positive direction. As shown in Scheme 1, the major dehydrated products identified are isosorbide, 1,4-sorbitan and a very small amount of 3,6-sorbitan. 2,5-Anhydro-D-sorbitol, 1,5-anhydro-D-sorbitol and some non-identified substances were all termed here as “others”. To compare different catalysts, we chose various acid catalysts such as H2SO4, Amberlyst-15 resin, Hβ and HZSM-5 zeolites. The results listed in Table 2 show that Hβ (Si/Al = 25) and HZSM-5 (Si/Al = 40) give a relatively low sorbitol conversion and yield of isosorbide. Both Amberlyst-15 resin and SBA-15-SO3H have high reactivity, but yield of isosorbide is low. Compared with those solid catalysts, the SA-SiO2-60.5 catalyst exhibits the highest sorbitol conversion (100%) and yield of isosorbide (84%). This reactivity is very close to that of traditional homogeneous catalysts such as H2SO4.| Entry | Catalyst | Sorbitol conv. (%) | Yield (%) | Othersb (%) | |
|---|---|---|---|---|---|
| Isosorbide | Sorbitan | ||||
| a Reaction conditions: 120 °C, 10 h, 1000 Pa, 50 g of sorbitol, 1.0 g of catalyst.b The by-products are 2,5-anhydro-D-sorbitol, 1,5-anhydro-D-sorbitol, and some others.c Undetectable.d After ten recycles. | |||||
| 1 | SA-SiO2-60.5 | 100 | 84 | 11 | 5 |
| 2 | H2SO4 | 100 | 90 | —c | 7 |
| 3 | Amberlyst-15 | 93 | 71 | 5 | 24 |
| 4 | SBA-15-SO3H | 95 | 70 | —c | 11 |
| 5 | Hβ (25) | 69 | 25 | 22 | 22 |
| 6 | HZSM-5 (40) | 30 | 9 | 17 | 4 |
| 7 | MCM-49 (30) | 73 | —c | 29 | 14 |
| 8 | SA-SiO2-60.5d | 100 | 75 | 20 | 5 |
| 9 | SBA-15-SO3Hd | 77 | 31 | 37 | 9 |
It is worth noting that although SA-SiO2-60.5 does not offer the maximum acidity, it displays good reaction performance (see Table 2). Compared with other reported hydrophilic catalysts in Table S2,† SA-SiO2-60.5 provides a higher yield of isosorbide and the reaction condition is moderate. This should be attributed to its suitable large pore size and high surface area (see Table 1). Thus it is beneficial to the mass transfer process, thereby inhibiting coke formation.
Fig. 5 shows the activity for the dehydration of sorbitol over various SA-SiO2-X at 120 °C for 10 h. With increasing MPTS loading from 10.6% to 40.9%, the yield of isosorbide increases obviously. When the MPTS loading increases from 40.9% to 60.5%, the growth rate of isosorbide yield slows down. Thereafter, with a continued increase of MPTS loading, the yield of isosorbide decreases. Yield of isosorbide and conversion of sorbitol are maximized at 60.5% MPTS loading. This phenomenon suggests that the further increase of MPTS loading (above 60.5%) will physically block the pores of micro-bead silica.40 Thus this seriously obstructs the internal diffusion and transfer of reactants and decreases the yield of isosorbide.
 | ||
| Fig. 5 Effect of different MPTS loadings on the performance in sorbitol dehydration. Reaction conditions: 50 g sorbitol, 1.0 g catalyst, 120 °C, 10 h. | ||
The influence of reaction temperature on the dehydration reaction was investigated, and the results are shown in Fig. 6. As shown in Fig. 6a, the conversion of sorbitol is 100% at 100–160 °C. At 100 °C, the yield of isosorbide is low, ca. 60%. On increasing the dehydration reaction temperature to 120 °C, the yield of isosorbide reaches the highest value (84%), and then decreases with further increasing the temperature to 160 °C (see Fig. 6b), since more coke during the test is formed. But the sorbitan yield, a main intermediate product,29,46 decreases as the temperature increases (see Fig. 6c). These results demonstrate that an appropriate reaction temperature was crucial for achieving a high yield of isosorbide.47 The highest catalytic performance is observed at 120 °C for 10 h under vacuum with 2% SA-SiO2-60.5 (100% conversion of sorbitol and 84% yield of isosorbide).
Recyclability is of great importance for applying a solid catalyst in industrial production. After reaction, the SA-SiO2-60.5 was washed in ultrasonic pool with water and glycol dimethyl ether to evaluate its recyclability, and the results are shown in Fig. 7. After being reused 10 times, sorbitol conversion is still 100% and the yield of isosorbide decreases only slightly. Compared with other zeolites,48 the regeneration process does not require calcination and the synthesis does not need surfactant or template so that the cost can be reduced. All the results indicate that SA-SiO2-60.5 exhibits excellent stability and is of low cost.
As shown in Table S2,† most of the hydrophilic catalysts such as oxides, phosphates and sulfated materials showed a relatively high reactivity compared with other hydrophobic catalysts, which makes us believe that the high reactivity and stability of SA-SiO2-60.5 are derived from its high surface hydrophilicity. As observed in Fig. 8, the sorbitol and isosorbide contact angles on SA-SiO2-60.5 are about 12.1° and 34.5°, respectively. This indicates that SA-SiO2-60.5 has a highly hydrophilic surface, and its surface affinity to sorbitol is stronger than that to isosorbide. Scheme 3 shows the process of sorbitol dehydration to isosorbide. The high surface hydrophilicity and different surface affinity of SA-SiO2-60.5 catalyst are in favor of the feed (sorbitol) reaching and the product (isosorbide) leaving active sites rapidly, thus suppressing the sequential reactions which cause coke formation. Under the reaction conditions (100–160 °C, 650–1000 Pa), water, formed in the reaction, can be removed from the reactor instantly, although it is a more polar molecule than sorbitol and isosorbide. This avoids the competitive adsorption of water on the surface of the catalyst with high hydrophilicity. As a regeneration treatment in each cycle, the catalyst was washed with water and glycol dimethyl ether. Moreover, the high surface hydrophilicity of SA-SiO2-60.5 catalyst enables the deposited coke, which is hydrophobic, to be washed away easily. So the catalyst activity is restored. Therefore, the physical and chemical properties of SA-SiO2-60.5 are also stable. For example, after recycling ten times, SA-SiO2-60.5 still gave acid site at 790 μmol g−1, which is slightly lower than that of the fresh catalyst (840 μmol g−1). On the contrary, the acid site of SBA-15-SO3H almost decreased to zero, and its recyclability was very poor. From the TGA (Fig. S1†) and FT-IR curves (Fig. 1b), the curves of SA-SiO2-60.5 remain almost unchanged after ten recycles. It can be concluded that SA-SiO2-60.5 is easily regenerated and exhibits good stability.
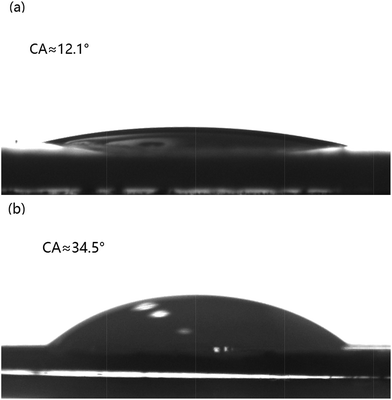 | ||
| Fig. 8 Sorbitol droplet contact angle (CA) on SA-SiO2-60.5 (a) and isosorbide droplet CA on SA-SiO2-60.5 (b). | ||
Conclusions
We have found that SA-SiO2-60.5 is a highly effective solid acid catalyst for dehydration of sorbitol into isosorbide in solvent-free condition. The used catalyst can be easily regenerated by washing with water and glycol dimethyl ether. And the catalyst displays good stability because it can be reused 10 times without significant loss of activity and selectivity. The excellent performance of SA-SiO2-60.5 is attributed to its suitable large pore diameter and high surface hydrophilicity that is beneficial to feed adsorption and product desorption, and inhibits the deposition of coke. Thus, the SA-SiO2 catalyst prepared in this work has high potential for use in the conversion of the most abundant biomass into platform chemicals.Acknowledgements
This work was supported by Jiangsu province science & technology innovation project (BY2014037-12), and the project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.References
- K. Saravanan, B. Tyagi, R. S. Shukla and H. C. Bajaj, Appl. Catal., B, 2015, 172–173, 108–115 CrossRef CAS.
- L. Wang, D. Li, H. Watanabe, M. Tamura, Y. Nakagawa and K. Tomishige, Appl. Catal., B, 2014, 150–151, 82–92 CrossRef CAS.
- N. Li, G. A. Tompsett, T. Zhang, J. Shi, C. E. Wyman and G. W. Huber, Green Chem., 2011, 13, 91–101 RSC.
- Y. Yang, C.-w. Hu and M. M. Abu-Omar, Green Chem., 2012, 14, 509–513 RSC.
- Y. Liu, L. Chen, T. Wang, Y. Xu, Q. Zhang, L. Ma, Y. Liao and N. Shi, RSC Adv., 2014, 4, 52402–52409 RSC.
- P. Gallezot, Top. Catal., 2010, 53, 1209–1213 CrossRef CAS.
- T. Deng, X. Cui, Y. Qi, Y. Wang, X. Hou and Y. Zhu, Chem. Commun., 2012, 48, 5494–5496 RSC.
- C.-H. Zhou, X. Xia, C.-X. Lin, D.-S. Tong and J. Beltramini, Chem. Soc. Rev., 2011, 40, 5588–5617 RSC.
- B. Zhang, Y. Zhu, G. Ding, H. Zheng and Y. Li, Green Chem., 2012, 14, 3402–3409 RSC.
- H. Kobayashi, H. Matsuhashi, T. Komanoya, K. Hara and A. Fukuoka, Chem. Commun., 2011, 47, 2366–2368 RSC.
- M. Liu, W. Deng, Q. Zhang, Y. Wang and Y. Wang, Chem. Commun., 2011, 47, 9717–9719 RSC.
- Y. Morita, S. Furusato, A. Takagaki, S. Hayashi, R. Kikuchi and S. T. Oyama, ChemSusChem, 2014, 7, 748–752 CrossRef CAS PubMed.
- S. Van de Vyver, J. Geboers, M. Dusselier, H. Schepers, T. Vosch, L. Zhang, G. Van Tendeloo, P. A. Jacobs and B. F. Sels, ChemSusChem, 2010, 3, 698–701 CrossRef CAS PubMed.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–554 RSC.
- H. Kobayashi and A. Fukuoka, Green Chem., 2013, 15, 1740–1763 RSC.
- H.-J. L. Gwi-Taek Jeong, H.-S. Kim and D.-H. Park, Appl. Biochem. Biotechnol., 2006, 129, 265–277 CrossRef.
- J. Xia, D. Yu, Y. Hu, B. Zou, P. Sun, H. Li and H. Huang, Catal. Commun., 2011, 12, 544–547 CrossRef CAS.
- M. Rose and R. Palkovits, ChemSusChem, 2012, 5, 167–176 CrossRef CAS PubMed.
- P. Sun, X. Long, H. He, C. Xia and F. Li, ChemSusChem, 2013, 6, 2190–2197 CrossRef CAS PubMed.
- A. Yamaguchi, N. Hiyoshi, O. Sato and M. Shirai, Green Chem., 2011, 13, 873–881 RSC.
- G. Flèche and M. Huchette, Starch - Stärke, 1986, 38, 26–30 CrossRef.
- A. Yamaguchi, O. Sato, N. Mimura and M. Shirai, RSC Adv., 2014, 4, 45575–45578 RSC.
- A. Kamimura, K. Murata, Y. Tanaka, T. Okagawa, H. Matsumoto, K. Kaiso and M. Yoshimoto, ChemSusChem, 2014, 7, 3257–3259 CrossRef CAS PubMed.
- N. A. Khan, D. K. Mishra, I. Ahmed, J. W. Yoon, J.-S. Hwang and S. H. Jhung, Appl. Catal., A, 2013, 452, 34–38 CrossRef CAS.
- A. A. Dabbawala, D. K. Mishra and J.-S. Hwang, Catal. Commun., 2013, 42, 1–5 CrossRef CAS.
- X. Zhang, D. Yu, J. Zhao, W. Zhang, Y. Dong and H. Huang, Catal. Commun., 2014, 43, 29–33 CrossRef.
- J. Li, A. Spina, J. A. Moulijn and M. Makkee, Catal. Sci. Technol., 2013, 3, 1540–1546 CAS.
- O. A. Rusu, W. F. Hoelderich, H. Wyart and M. Ibert, Appl. Catal., B, 2015, 176–177, 139–149 CrossRef CAS.
- R. M. de Almeida, J. Li, C. Nederlof, P. O'Connor, M. Makkee and J. A. Moulijn, ChemSusChem, 2010, 3, 325–328 CrossRef PubMed.
- J. Li, W. Buijs, R. J. Berger, J. A. Moulijn and M. Makkee, Catal. Sci. Technol., 2014, 4, 152–163 CAS.
- N. A. Khan, D. K. Mishra, J.-S. Hwang, Y.-W. Kwak and S. H. Jhung, Res. Chem. Intermed., 2011, 37, 1231–1238 CrossRef CAS.
- Y. Xiu, A. Chen, X. Liu, C. Chen, J. Chen, L. Guo, R. Zhang and Z. Hou, RSC Adv., 2015, 5, 28233–28241 RSC.
- J. Zhang, L. Wang, F. Liu, X. Meng, J. Mao and F.-S. Xiao, Catal. Today, 2015, 242, 249–254 CrossRef CAS.
- M. A. Z. P. Salehi, F. Shirinic and M. Baghbanzadeh, Curr. Org. Chem., 2006, 10, 2171–2189 CrossRef.
- Z. Hasan and S. H. Jhung, Eur. J. Inorg. Chem., 2014, 2014, 3420–3426 CrossRef CAS.
- A. A. Dabbawala, J. J. Park, A. H. Valekar, D. K. Mishra and J.-S. Hwang, Catal. Commun., 2015, 69, 207–211 CrossRef CAS.
- W. N. P. van der Graaff, K. G. Olvera, E. A. Pidko and E. J. M. Hensen, J. Mol. Catal. A: Chem., 2014, 388–389, 81–89 CrossRef CAS.
- M. H. Valkenberg, C. deCastro and W. F. Holderich, Green Chem., 2002, 4, 88–93 RSC.
- Z. W. Y. Huang, Y. Liu and Q. Ren, J. Chem. Eng. Chin. Univ., 2008, 22, 721–724 Search PubMed.
- J. D. Y. Zhang, Y. Shan, Y. Zhou, G. Wang and M. Li, J. Chem. Eng. Chin. Univ., 2010, 24, 825–829 CrossRef.
- W. Zhou, J. Liu, J. Pan, F. a. Sun, M. He and Q. Chen, Catal. Commun., 2015, 69, 1–4 CrossRef CAS.
- B. Xue, J. Xu, C. Xu, R. Wu, Y. Li and K. Zhang, Catal. Commun., 2010, 12, 95–99 CrossRef CAS.
- Y. Guo, K. Li, X. Yu and J. H. Clark, Appl. Catal., B, 2008, 81, 182–191 CrossRef CAS.
- S. Hamoudi and S. Kaliaguine, Microporous Mesoporous Mater., 2003, 59, 195–204 CrossRef CAS.
- C. Pirez, A. F. Lee, J. C. Manayil, C. M. A. Parlett and K. Wilson, Green Chem., 2014, 16, 4506–4509 RSC.
- N. Li and G. W. Huber, J. Catal., 2010, 270, 48–59 CrossRef CAS.
- R. Otomo, T. Yokoi and T. Tatsumi, Appl. Catal., A, 2015, 505, 28–35 CrossRef CAS.
- H. Kobayashi, H. Yokoyama, B. Feng and A. Fukuoka, Green Chem., 2015, 17, 2732–2735 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra27510e |
| This journal is © The Royal Society of Chemistry 2016 |









