Nanospheres based on PLGA/amphiphilic cyclodextrin assemblies as potential enhancers of Methylene Blue neuroprotective effect†
Abstract
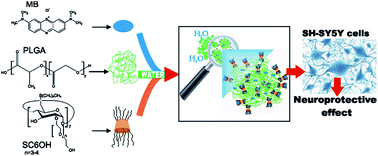
Methylene Blue (MB) has recently showed beneficial effects towards neurological disorders such as Alzheimer’s and Parkinson’s diseases. Intravenous administration of MB could be difficult because of its poor cooperation with patients, thus entrapment in a carrier system could improve compliance. PLGA nanospheres have been proposed as a delivery system for MB, but they suffer from low encapsulation efficiency and rapid release of their cargo. Here, we design nanospheres with high affinity for hydrophilic MB based on PLGA and a non-ionic amphiphilic cyclodextrin (SC6OH) as an additional component. Interaction between MB and SC6OH was firstly investigated by UV-vis spectroscopy and steady-state emission fluorescence in aqueous solution. PLGA/SC6OH nanospheres loaded with MB were prepared by a nanoprecipitation/solvent displacement method and characterized by STEM and FTIR-ATR analysis. They display sizes of about 200 nm, and a higher encapsulation efficiency with respect to PLGA nanospheres prepared without SC6OH. This latter modulates the release profiles of MB from the nanospheres, producing a release sustained for five days. In vitro biological studies on human neuroblastoma SH-SY5Y cells demonstrated that PLGA/SC6OH nanospheres did not affect cell viability. In addition, MB loaded-PLGA/SC6OH nanospheres produced significant neuroprotection against the metabolic effects of iodoacetic acid, especially in the presence of NADH electron donor.

 Please wait while we load your content...
Please wait while we load your content...