Silicon dioxide@graphene oxide-graft-poly(γ-benzyl-l-glutamate) as an advanced hybrid nanofiller reinforces poly(l-lactide)†
Abstract
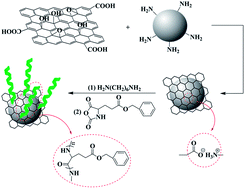
A critical challenge in the preparation of reinforced polymer nanocomposites is to prevent the aggregation of nanofillers. In this work, a novel poly(γ-benzyl-L-glutamate) (PBLG)-modified silicon dioxide@graphene oxide nanofiller (SiO2@GO-g-PBLG) was prepared via a continuous electrostatic complex and ring-opening polymerization (ROP) method. The grafted PBLG prevented the aggregation of SiO2 nanoparticles and GO sheets, realized the colloid stability of SiO2@GO-g-PBLG in organic solvents, and thus increased its phase interaction in a polymer matrix. The hybrid nanofiller greatly enhanced the mechanical properties of poly(L-lactide) (PLLA). As a typical feature, the tensile strength of PLLA nanocomposites with only 5 wt% of SiO2@GO-g-PBLG was 88.9 MPa, about 45% higher than that of pure PLLA. In addition, the hybrid nanofillers also have some positive effects on improving the thermal stability of PLLA. Therefore, SiO2@GO-g-PBLG was a promising hybrid nanofiller to reinforce polymers such as PLLA.

 Please wait while we load your content...
Please wait while we load your content...