Synthesis of protein/hydroxyapatite nano-composites by a high-gravity co-precipitation method
H. Penga,
D. Zhangab,
B. Sunab,
Y. Luob,
S. Lv*a,
J. Wang*ab and
J. Chen*ab
aState Key Laboratory of Organic-inorganic Composites, Beijing University of Chemical Technology, 15 Beisanhuan East Road, Chaoyang District, Beijing, China 100029. E-mail: lvshanshan@mail.buct.edu.cn; Tel: +86 10 64411656
bResearch Center of the Ministry of Education for High Gravity Engineering & Technology, Beijing University of Chemical Technology, 15 Beisanhuan East Road, Chaoyang District, Beijing, China 100029. E-mail: wangjx@mail.buct.edu.cn; Fax: +86 10 64434784; Tel: +86 10 64447274
First published on 22nd January 2016
Abstract
This study explored potential use of a high-gravity co-precipitation strategy to synthesize gelatin/hydroxyapatite (Gel/HAP) nano-composites with high protein adsorption efficiencies. The physicochemical properties were characterized using X-ray diffraction, transmission electron microscopy, Fourier transform infrared spectra, thermal gravimetric analysis and Brunauer–Emmett–Teller. Biocompatibility was determined by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assays on hFOB 1.19 osteoblast cells. The results confirmed formation of Gel/HAP composites exhibiting nano-rod-like morphologies with high protein adsorption efficiencies and biocompatibilities. In addition, the Gel/HAP nano-composites were molded into cylindrical shaped Calcium Phosphate Cements (CPC), displaying an average compressive modulus of ∼0.6 GPa. The compressive modulus was comparable with that of a human cancellous bone, suggestive of great potential of the Gel/HAP nano-composites in various biomedical applications through serving as bone substitute materials. The high-gravity co-precipitation technique was also applied to synthesize Silk/HAP nano-composites. Therefore, the high-gravity co-precipitation method provides a novel approach for synthesis of various protein/HAP materials.
Introduction
There is a great demand for synthetic bone substitute materials because of various diseases.1,2 Considering that natural bones are composites of mainly hydroxyapatite (HAP) nano-crystals embedded in collagen (Col) fibrils,3 research interest has focused on fabrication of bone-mimetic nano-composites for bone substitute applications, such as Col/HAP.4 Due to low solubility and expensive price of Col, gelatin (Gel), a denatured form of Col, has been a favoured substitution of Col. With respect to Col, Gel has many advantages such as inexpensive, commercially available, dispersible in water, easy for chemical modification, nontoxic, completely resorbable in vivo, low immunogenicity and FDA approval. Hence, Gel/HAP composites have received considerable attention and been extensively investigated as promising bone replacement biomaterials for hard tissue repairing.5–11Many researchers have carried out various strategies to prepare Gel/HAP composites.12–16 Gel/HAP composites have been prepared by dispersing HAP powders in Gel solutions. A major disadvantage of this method was that the HAP could not disperse well in Gel and would aggregate.17,18 To overcome the disadvantage, researchers developed biomimetic processes to prepare Gel/HAP composites, by co-precipitation of HAP in Gel solutions, very similar to biological formation of natural bones.19,20 Conventionally, the co-precipitation involved gradually adding small droplets of phosphate salt solution into a bulk solution containing pre-mixed Gel and calcium salts. However, the addition of a small droplet into a bulk solution might cause a heterogeneous distribution of the phosphate groups, which would usually make an unfavourable long period of continuously and vigorously stirring necessary to ensure thorough reactions. In addition, protein adsorption on inorganic materials has always been a complex paradigm,21 which made Gel adsorption on HAP a difficult process. As a result, a large amount of Gel was washed away, leading to lower protein adsorption efficiencies than theoretical values.
To encounter these problems, we devoted to develop a new rapid method for synthesis of Gel/HAP nano-composites with high protein adsorption efficiencies. High-gravity (HG) technology has been an effective chemical engineering method to intensify mass transfer in multiphase systems since 1979.22 The general idea of the HG technology is to strongly intensify substance micro-mixing under a HG environment in a rotating packed bed (RPB). In a typical HG experiment, liquid flows are pumped into the equipment and can be accelerated at a large rotating speed by the RPB. The HG process provides a strong shear stress – a centrifugal force that would split reactant solutions into tiny liquid droplets to enable better contact of the reagents and more thorough reactions between the reagents. In this way, the mass transfer between the liquid–liquid for mixing or adsorption will be largely enhanced.22 Therefore, we hypothesized that the HG technology could give better Gel adsorption performance in co-precipitation than conventional co-precipitation in stirred tank reactors (STR).
To prove our hypothesis, in this study we explored the potential of the HG technology in co-precipitation preparation of Gel/HAP nano-composites to enhance the protein adsorption. Gel/HAP nano-composites were synthesized under conventional co-precipitation in STR and under a HG field. We characterized and compared physicochemical and biocompatible properties of the Gel/HAP nano-composites under both conditions. Further, we explored potential of the Gel/HAP nano-composites in preparing Calcium Phosphate Cements (CPC), which is one of the most common materials in bone substitute applications. Moreover, we explored possibility of applying the HG co-precipitation technique to other systems, such as silk protein/hydroxyapatite (Silk/HAP) nano-composites.
Experimental
Materials and experimental setup
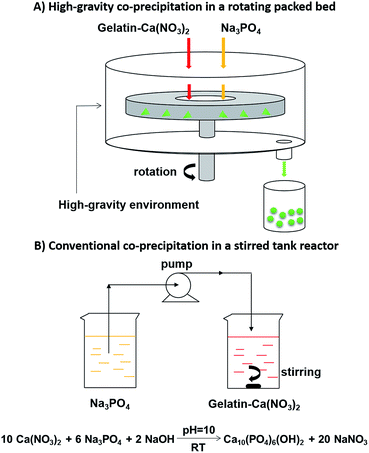
A schematic of experimental setup for the high-gravity (HG) co-precipitation process was shown in Fig. 1. The rotating packed bed (RPB) have been described previously and can be found elsewhere.23 The RPB mainly consisted of a rotor, a casing, two liquid inlets and a liquid outlet. Stainless wire mesh packing was installed inside the rotor. The casing has an inner diameter of 35 mm, an outer diameter of 105 mm, and an axial length of 35 mm.Analytical reagent grade calcium nitrate tetrahydrate (Ca(NO3)2·4H2O), sodium phosphate (Na3PO4), sodium hydroxide (NaOH) and absolute ethyl alcohol (CH3CH2OH) were purchased from Sinopharm Chemical Reagent Beijing Co. Ltd. Gelatin (powder from cold water fish) was purchased from Sigma-Aldrich, and used without further purification.
Raw silk (produced in Zhejiang, China) cut into small pieces were boiled in 0.05 wt% Na2CO3 for 30 min at 90 °C and washed thoroughly with deionized water for 3 times to extract natural sericin coatings. The degummed silk was dried overnight at room temperature. The dried silk was then dissolved in a Ca(NO3)2–ethanol–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) solution at 60 °C for 2 h. After dissolution, the silk was centrifuged at 8000 rpm for 10 min at 4 °C to remove aggregates; the supernatant was continuously dialyzed for 3 days with water changed every 5–7 h using a cellulose dialysis membrane (MWCO 8000 Da) to remove salts and any other remaining impurities from the solution. The dialyzed silk solution was then lyophilized to obtain regenerated silk protein.24
8) solution at 60 °C for 2 h. After dissolution, the silk was centrifuged at 8000 rpm for 10 min at 4 °C to remove aggregates; the supernatant was continuously dialyzed for 3 days with water changed every 5–7 h using a cellulose dialysis membrane (MWCO 8000 Da) to remove salts and any other remaining impurities from the solution. The dialyzed silk solution was then lyophilized to obtain regenerated silk protein.24
Preparation of protein/hydroxyapatite
In a typical HG co-precipitation reaction, 2 L of aqueous solution containing 12.5 g of gelatin (Gel) and 0.1 M Ca(NO3)2 (Gel–Ca(NO3)2, pH = 10) was prepared. An aqueous solution of Na3PO4 (0.1 M, pH = 10) was also prepared. The two solutions were simultaneously pumped into the RPB from different inlets at a HG level of 1098 m s−2 (rotational speed at 1600 rpm (RPB1600)). The flow rates of the two solutions were set to give a Ca/P molar ratio at 1.67 under the idealized stoichiometric equation:| 10Ca(NO3)2 + 6Na3PO4 + 2NaOH → Ca10(PO4)6(OH)2 + 20NaNO3 | (1) |
After entering the RPB, the two solutions vigorously mixed and reacted to form Gel/HAP. The Gel/HAP was collected, aged at room temperature overnight, filtered and washed with deionized water. The product was dried at 80 °C for 12 h, then ground into powder to obtain Gel/HAP composites (Gel/HAPRPB1600). Silk/HAP was prepared the same way as Gel/HAP by replacing Gel with the regenerated silk protein.
For comparison purposes, Gel/HAP composites were also prepared by a conventional co-precipitation method in a stirred tank reactor (STR) by a modified precipitation method.25 Aqueous solutions of Gel–Ca(NO3)2 and Na3PO4 were prepared in the same way as that in the HG co-precipitation reaction. The Na3PO4 solution was added into the Gel–Ca(NO3)2 solution drop by drop (4 mL min−1) under vigorously stirring. Volumes of the two solutions were calculated to give a Ca/P molar ratio at 1.67. After completing the addition of the Na3PO4 solution, the suspension was continually stirred for another 2 h to form Gel/HAP. The Gel/HAP was aged at room temperature overnight, filtered and washed with deionized water, followed by drying at 80 °C for 12 h and then grounding into powder to obtain Gel/HAP composites (Gel/HAPSTR).
Preparation of calcium phosphate cements (CPC)
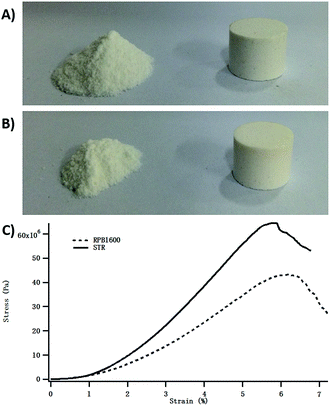
Both the Gel/HAPRPB1600 and Gel/HAPSTR were separately shaped into cylindrical samples of ∼13 mm in diameter and ∼10 mm in height using a powder tablet machine (FW-4A, TIAN JIN TUO PU INSTRUMENTS CO., LTD) under compression pressure of 5 MP for 5 min. The samples were removed from the cylindrical mold and used for mechanical tests.Physicochemical characterizations of Gel/HAP
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 and compressed (10 MPa) to form disks at room temperature for FTIR analysis, which was carried out on an FTIR instrument (FTIR, Bruker Optics VERTEX70, USA) in the transmission mode in a range of 400 to 4000 cm−1 with a resolution of 4 cm−1.
100 and compressed (10 MPa) to form disks at room temperature for FTIR analysis, which was carried out on an FTIR instrument (FTIR, Bruker Optics VERTEX70, USA) in the transmission mode in a range of 400 to 4000 cm−1 with a resolution of 4 cm−1.| Gelatin (%) = weight loss of Gel/HAP composites − weight loss of HAP (%) | (2) |
Mechanical tests
Compressive tests were conducted to evaluate mechanical performance of the Gel/HAP-based CPC. The tests were carried out with an Instron-1185 mechanical testing machine with a load cell of 10 kN at a cross-head speed of 2 mm min−1 (as required by GB8813-88 ISO844-1978) until failure. Compressive moduli were calculated from slope of the initial linear portion of the stress–strain curves at 5% of strain. For experimental convenience, all tests were done at room temperature.Cell culture and MTT-based cytotoxicity assay
Osteoblast cells (hFOB 1.19) were purchased from Xiehe Cell Resource Center (Xiehe, Beijing). The cells were cultured according to the recommended protocol in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of Ham's F12 Medium Dulbecco's Modified Eagle's Medium (Xiehe), with 2.5 mM L-glutamine, supplemented with 0.3 mg mL−1 G418 (Xiehe) and 10% fetal bovine serum (Xiehe). Cells were maintained at 34 °C with 5% CO2, fed every 2–3 days and passaged according to the protocol.
1 mixture of Ham's F12 Medium Dulbecco's Modified Eagle's Medium (Xiehe), with 2.5 mM L-glutamine, supplemented with 0.3 mg mL−1 G418 (Xiehe) and 10% fetal bovine serum (Xiehe). Cells were maintained at 34 °C with 5% CO2, fed every 2–3 days and passaged according to the protocol.
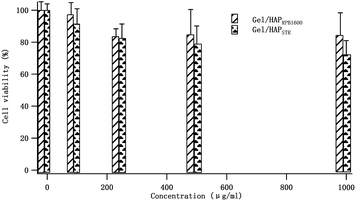
To assess cytotoxicity of the Gel/HAP, hFOB 1.19 cells were seeded in a standard 96-well plate at a density of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well (100 μL) in triplicate. After 24 h, various amounts of the Gel/HAP were added into the wells to give final concentrations of up to 1000 μg mL−1. Cells were incubated for another 24 h, then used in the cytotoxicity assay. For the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay, 50 μL of the MTT solution (2.5 mg mL−1) was added to each experimental well, including controls (cells seeded without addition of the Gel/HAP). The plate was then returned to the cell culture incubator. After 4 h of incubation, the wells were aspirated, followed by addition of 150 μL of dimethyl sulfoxide (DMSO) to each well. The plate cover was then removed and absorbance in each well was measured at 570 nm using a microtiter plate reader. The average absorbance values from triplicate readings were determined. Cell viability was calculated following
000 cells per well (100 μL) in triplicate. After 24 h, various amounts of the Gel/HAP were added into the wells to give final concentrations of up to 1000 μg mL−1. Cells were incubated for another 24 h, then used in the cytotoxicity assay. For the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay, 50 μL of the MTT solution (2.5 mg mL−1) was added to each experimental well, including controls (cells seeded without addition of the Gel/HAP). The plate was then returned to the cell culture incubator. After 4 h of incubation, the wells were aspirated, followed by addition of 150 μL of dimethyl sulfoxide (DMSO) to each well. The plate cover was then removed and absorbance in each well was measured at 570 nm using a microtiter plate reader. The average absorbance values from triplicate readings were determined. Cell viability was calculated following
| Cell viability (%) = (ABSe − ABSb)/(ABS0 − ABSb) × 100 | (3) |
Statistical analysis
All data were presented as means plus or minus standard deviation arising from measurements made from at least three independent experiments. Statistical analysis was performed on cell data using an unpaired Student's t-test with a confidence level of 0.05 unless otherwise noted.Results and discussions
Preparation of gelatin/hydroxyapatite (Gel/HAP) nano-composites
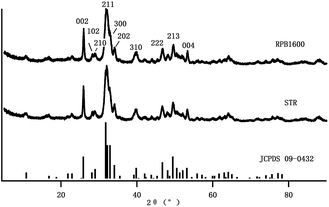
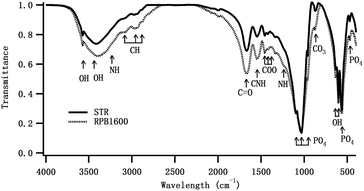
Ca(NO3)2 and Na3PO4 were used as calcium and phosphorous sources, respectively. Gel was dissolved in the Ca(NO3)2 solution to get a pre-mixed solution of Gel and Ca(NO3)2 (Gel–Ca(NO3)2). A schematic of the experimental setup for the high-gravity (HG) co-precipitation was shown in Fig. 1. The principle component was a rotating packed bed (RPB). The two reactant solutions of Gel–Ca(NO3)2 and Na3PO4 were simultaneously pumped into the RPB from different inlets. The solutions were spread and split into tiny liquid droplets, vigorously mixed and reacted, via a centrifugal force generated by the RPB. In this way, the HG co-precipitation enabled better mixing and contact of the reagents, leading to more thorough reactions between the reagents than conventional co-precipitation in stirred tank reactors (STR).22,26,27 The Gel/HAP composites synthesized by the HG co-precipitation were labelled as Gel/HAPRPB1600. Gel/HAP composites were also prepared by a conventional co-precipitation method to serve as a control (Gel/HAPSTR).Characterizations of the Gel/HAP nano-composites
Use of the Gel/HAPRPB1600 in preparing calcium phosphate cements (CPC)
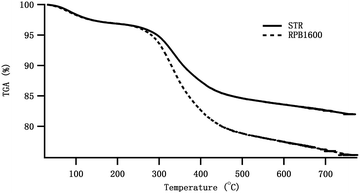
The excellent cytocompatibility implied great potential of using the Gel/HAPRPB1600 in biomedical applications. To explore possibility of applying the Gel/HAPRPB1600 as bone substitute materials, we used the Gel/HAPRPB1600 in preparing CPC, which were one of the most common bone substitute materials.39,40 The Gel/HAPRPB1600 were molded into cylindrical samples and characterized in mechanical tests (Fig. 7). Compressive modulus of the Gel/HAPRPB1600-based CPC was measured to be 0.6 ± 0.1 GPa (n = 4) at 5% strain. The compressive modulus of the Gel/HAPRPB1600-based CPC was comparable to that of natural trabecular bones,31 which demonstrated great potential of the Gel/HAPRPB1600 in fabricating new materials such as bone fillers.41 Gel/HAPSTR-based CPC were also prepared, displaying an average compressive modulus of 1.1 ± 0.1 GPa (n = 4). The compressive modulus of the Gel/HAPSTR-based CPC was higher than that of the Gel/HAPRPB1600-based CPC, which might be explained by the lower Gel content in the Gel/HAPSTR as indicated by the TEM, FTIR and TGA results. It has been reported that compressive modulus of HAP would be significantly enhanced upon addition of Gel. Gel could serve as “glue” that bonds HAP. But the enhancement effect did not increasing linearly with increasing the amount of Gel. Although presence of up to 5% of Gel could improve mechanical properties of Gel/HAP composites, Gel contents greater than 5% would decrease the compressive strength.30To prove our explanation, we investigated the effect of Gel contents on the mechanical properties of Gel/HAPRPB1600-based CPC. More Gel/HAPRPB1600-based CPC were prepared by tuning the initial Gel contents (0%, 10%, 20%, 30% and 38%). As shown in Fig. 8, the compressive moduli of Gel/HAPRPB1600-based CPC with different initial Gel content were all larger than that of CPC without Gel (0%). As the initial Gel content increased from 0% to 10%, the compressive modulus increased. However, as the initial Gel content further increased from 10% to 38%, the compressive modulus decreased. The initial Gel content of 10% was found to be the optimal for preparing Gel/HAPRPB1600-based CPC. Because the initial Gel content could not represent the precise Gel content, we calculated Gel adsorption efficiencies from TGA analysis results. As Fig. 9 showed, the optimal initial Gel content of 10% corresponded to an efficiency of ∼5%, which was consistent with previous report.30 Beside of proving our explanation, it is also interesting to notice that the (initial content)–(adsorption efficiency) relation could be described reasonably well by a linear fit (Fig. 9), suggesting an intrinsic relation between the initial Gel content and Gel adsorption efficiency under HG fields.
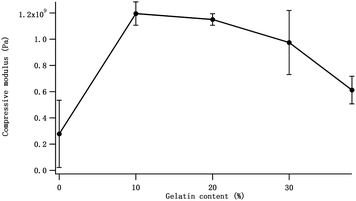 | ||
| Fig. 8 The effect of the initial Gel content on the compressive modulus of the Gel/HAPRPB1600-based CPC. | ||
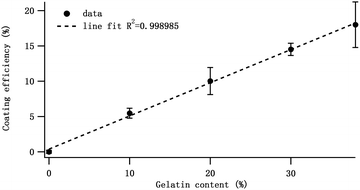 | ||
| Fig. 9 The Gel adsorption efficiency of the Gel/HAPRPB1600 prepared at different initial Gel content. The function could be fitted to a linear equation with R2 = 0.998985. | ||
Utilization of the HG co-precipitation in synthesizing silk protein/hydroxyapatite (Silk/HAP) nano-composites
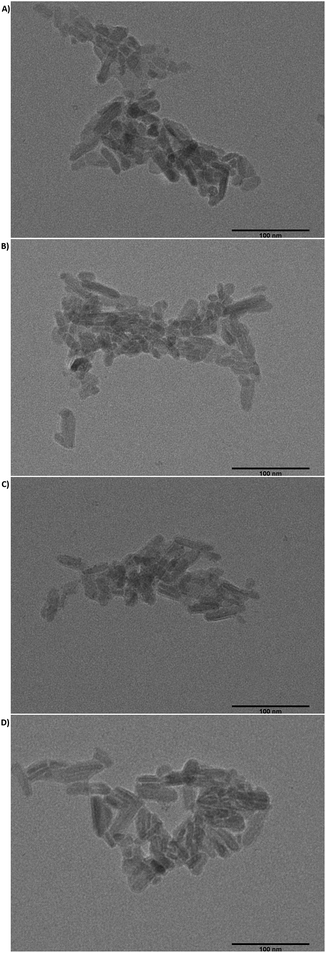
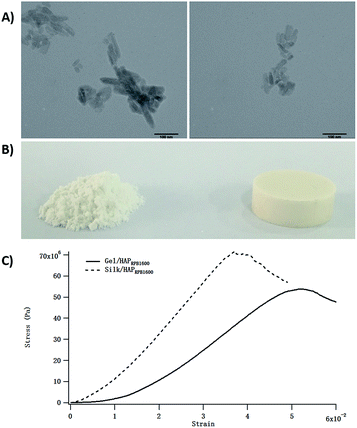
After we explored the potential of the HG co-precipitation in synthesizing Gel/HAP nano-composites, we went a step further to explore whether this technology could be applied to other systems. Among various proteins used in HAP-based composites, silk protein is one of the most extensively studied. Herein, we used a model system-Silk/HAP. Silk/HAP was prepared the same way as Gel/HAPRPB1600 by replacing Gel with the regenerated silk protein. TEM results clearly demonstrated successful production of Silk/HAP nano-composites (Fig. 10A). The Silk/HAP displayed diameters and lengths between 4.7–27.0 and 19.0–105.8 nm, respectively. The Silk/HAP nano-composites were also molded into cylindrical CPC (Fig. 10B). The compressive modulus at 5% strain calculated from stress–strain curves (Fig. 10C) was found to be 1.2 ± 0.2 GPa.Conclusions
This study explored the potential use of a high-gravity (HG) co-precipitation method to prepare gelatin/hydroxyapatite (Gel/HAP) nano-composites with high protein adsorption efficiencies. Nano-rod shaped Gel/HAP composites with a diameter distribution of 3.3–18.2 nm and a length distribution of 9.4–47.7 nm were successfully obtained. A high Gel adsorption efficiency over that in the conventional co-precipitation was observed. Enhanced biocompatibilities with hFOB 1.19 osteoblast cells were also observed. The compressive modulus of Calcium Phosphate Cements (CPC) based on the resultant Gel/HAP nano-composites was ∼0.6 GPa, comparable to that of trabecular bones. These findings demonstrated great potential of the newly synthesized Gel/HAP nano-composites to be used as bone substitute materials for bone repairing. The high-gravity co-precipitation method provides a new approach for synthesis of Gel/HAP nano-composites and opens new possibilities for producing various other protein–inorganic nano-composite systems which require higher protein adsorption.Acknowledgements
The author would like to thank Dr Yuan Le and Mr Mengtao Liu for experimental support on the cell assay, thank Dr Zhiping Liu for help on the cement forming molds. The work is supported by the Foundation of Beijing University of Chemical Technology (12060029009, 12060046001), the Foundation of State Key Laboratory of Organic-inorganic Composites (201311, 201403002), the Fundamental Research Funds for the Central Universities (YS1401) and the National Natural Science Foundation of China (Grant no. 31400813).Notes and references
- J. D. Currey, Bones: structure and mechanics, Princeton University Press, Princeton [N.J.], 2nd edn, 2002 Search PubMed.
- S. Mann, Biomineralization: principles and concepts in bioinorganic materials chemistry, Oxford University Press, Oxford, 2001 Search PubMed.
- D. G. T. Strange and M. L. Oyen, Acta Biomater., 2011, 7, 3586–3594 CrossRef CAS PubMed.
- A. Tsetsekou, D. Brasinika, V. Vaou and E. Chatzitheodoridis, Mater. Sci. Eng., C, 2014, 43, 555–565 CrossRef CAS PubMed.
- A. Zaupa, A. T. Neffe, B. F. Pierce, A. Lendlein and D. Hofmann, Int. J. Artif. Organs, 2011, 34, 139–151 CrossRef CAS PubMed.
- A. Vyalikh, P. Simon, T. Kollmann, R. Kniep and U. Scheler, J. Phys. Chem. C, 2011, 115, 1513–1519 CAS.
- A. T. Neffe, A. Zaupa, B. F. Pierce, D. Hofmann and A. Lendlein, Macromol. Rapid Commun., 2010, 31, 1534–1539 CrossRef CAS.
- M. Dressler, E. Dombrowski, U. Simon, J. Bornstein, V. D. Hodoroaba, M. Feigl, S. Grunow, R. Gildenhaar and M. Neumann, J. Eur. Ceram. Soc., 2011, 31, 523–529 CrossRef CAS.
- G. Tronci, A. T. Neffe, B. F. Pierce and A. Lendlein, J. Mater. Chem., 2010, 20, 8875–8884 RSC.
- R. K. Brundavanam, Z.-T. Jiang, P. Chapman, X.-T. Le, N. Mondinos, D. Fawcett and G. E. J. g. p. m. e. a. Poinern, Ultrason. Sonochem., 2011, 18, 697–703 CrossRef CAS PubMed.
- K. Miura, T. Anada, Y. Honda, Y. Shiwaku, T. Kawai, S. Echigo, T. Takahashi and O. Suzuki, Appl. Surf. Sci., 2013, 282, 138–145 CrossRef CAS.
- K. J. Roche and K. T. Stanton, J. Cryst. Growth, 2015, 409, 80–88 CrossRef CAS.
- S. Teng and P. Wang, Mater. Lett., 2011, 65, 1348–1350 CrossRef CAS.
- J. H. An, O. S. Han, D. H. Kohn, Y. J. Park and H. J. Song, J. Nanosci. Nanotechnol., 2015, 15, 5668–5671 CrossRef CAS PubMed.
- S. N. Danilchenko, O. V. Kalinkevich, M. V. Pogorelov, A. N. Kalinkevich, A. M. Sklyar, T. G. Kalinichenko, V. Y. Ilyashenko, V. V. Starikov, V. I. Bumeyster, V. Z. Sikora and L. F. Sukhodub, J. Biomed. Mater. Res., Part A, 2011, 96, 639–647 CrossRef PubMed.
- Z. Su, J. Li, Z. Ouyang, M. M. L. Arras, G. Wei and K. D. Jandt, RSC Adv., 2014, 4, 14833–14839 RSC.
- M. Zandi, H. Mirzadeh, C. Mayer, H. Urch, M. B. Eslaminejad, F. Bagheri and H. Mivehchi, J. Biomed. Mater. Res., Part A, 2010, 92, 1244–1255 Search PubMed.
- M. Azami, M. Rabiee and F. Moztarzadeh, Polym. Compos., 2010, 31, 2112–2120 CrossRef CAS.
- B. Basu, S. K. Swain and D. Sarkar, RSC Adv., 2013, 3, 14622–14633 RSC.
- J. Lee, B. Choi, J. Choi, G. Kim, D. Hwang, M. Chang, J. Byun and U. Kim, Tissue Eng. Regener. Med., 2013, 10, 47–52 CrossRef CAS.
- V. O. Kollath, S. Mullens, J. Luyten, K. Traina and R. Cloots, RSC Adv., 2015, 5, 55625–55632 RSC.
- J. Chen, Y. Wang, F. Guo, X. Wang and C. Zheng, Ind. Eng. Chem. Res., 2000, 39, 948–954 CrossRef CAS.
- H. Peng, J. Wang, S. Lv, J. Wen and J. Chen, Ceram. Int., 2015, 41, 14340–14349 CrossRef CAS.
- H. Wang and Y. Zhang, Sci. Rep., 2014, 4 Search PubMed.
- W. Wei, R. Sun, Z. Jin, J. Cui and Z. Wei, Appl. Surf. Sci., 2014, 292, 1020–1029 CrossRef CAS.
- A. J. Nathanaela, S. I. Hong, D. Mangalaraj and P. C. Chen, Chem. Eng. J., 2011, 173, 846–854 CrossRef.
- Q. Yang, J. Wang, F. Guo and J. Chen, Ind. Eng. Chem. Res., 2010, 49, 9857–9863 CrossRef CAS.
- J. Chen and Y. Chang, Colloids Surf., B, 2011, 86, 169–175 CrossRef CAS PubMed.
- H. Wang, M. Bongio, K. Farbod, A. W. Nijhuis, J. van den Beucken, O. C. Boerman, J. C. van Hest, Y. Li, J. A. Jansen and S. C. Leeuwenburgh, Acta Biomater., 2014, 10, 508–519 CrossRef CAS PubMed.
- J. Chen and F. Chang, Appl. Surf. Sci., 2012, 262, 176–183 CrossRef CAS.
- S. K. Swain and D. Sarkar, Mater. Lett., 2013, 92, 252–254 CrossRef CAS.
- M. Azami, M. J. Moosavifar, N. Baheiraei, F. Moztarzadeh and J. Ai, J. Biomed. Mater. Res., Part A, 2012, 100, 1347–1355 CrossRef PubMed.
- Y. Han, S. Li, X. Cao, L. Yuan, Y. Wang, Y. Yin, T. Qiu, H. Dai and X. Wang, Sci. Rep., 2014, 4, 7 Search PubMed.
- M. Yin, Y. Yin, Y. Han, H. Dai and S. Li, J. Nanomater., 2014, 2014 Search PubMed.
- S. M. Zakaria, S. H. Sharif Zein, M. Othman and J. A. Jansen, J. Biomed. Mater. Res., Part A, 2013, 101, 1977–1985 CrossRef PubMed.
- J. Lee and H. Yun, J. Mater. Chem. B, 2014, 2, 1255–1263 RSC.
- A. J. Nathanael, R. Yuvakkumar, S. I. Hong and T. H. Oh, ACS Appl. Mater. Interfaces, 2014, 6, 9850–9857 CAS.
- C. Liao and J. Zhou, J. Phys. Chem. B, 2014, 118, 5843–5852 CrossRef CAS PubMed.
- M. Li, X. Liu, X. Liu and B. Ge, Clin. Orthop. Relat. Res., 2010, 468, 1978–1985 CrossRef PubMed.
- D. A. Oortgiesen, X. F. Walboomers, A. L. Bronckers, G. J. Meijer and J. A. Jansen, J. Tissue Eng. Regener. Med., 2014, 8, 202–209 CrossRef CAS PubMed.
- M. Peroglio, L. Gremillard, D. Eglin, P. Lezuo, M. Alini and J. Chevalier, Acta Biomater., 2010, 6, 3808–3812 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |