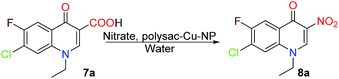An operational transformation of 3-carboxy-4-quinolones into 3-nitro-4-quinolones via ipso-nitration using polysaccharide supported copper nanoparticles: synthesis of 3-tetrazolyl bioisosteres of 3-carboxy-4-quinolones as antibacterial agents†
Chandra S. Azad and
Anudeep K. Narula*
and
Anudeep K. Narula*
“Hygeia” Centre of Excellence in Pharmaceutical Sciences (CEPS), GGS Indraprastha University, Sec. 16-C, Dwarka, New Delhi, India. E-mail: medchemlab58@gmail.com
First published on 4th February 2016
Abstract
Chitosan supported Cu nano-particles have been synthesized, and utilized for the synthesis of 3-nitro-4-quinolones from 3-carboxy-4-quinolones via ipso nitration. The synthesized 3-nitro derivatives of 4-quinolones were successfully converted into their 3-tetrazolyl bioisosteres which showed increased antibacterial activity as compared to the standard ciprofloxacin.
Major concerns have been raised about environmental impact, so remarkable exertions are being made towards the development of new environmentally friendly chemical processes with minimum pollutants.1 Heterogeneous catalysis offers a promising approach for the reduction of waste products, low contamination of the products with the active catalytic species, and separation (removal and recovery) and recycling of the catalyst.2 The immobilization of catalyst on a solid support is an advantageous method to improve the efficiency and ease of separation. Several successful examples are available for this kind of catalytic application.3 The important reported solid supports are inorganic materials (e.g. SiO2, Al2O3, ZrO2, TiO2 and MgF2), zeolite, charcoal, magnetic materials (Fe3O4 and Co) and polymers.4 The search for more environmentally friendly solid supports is being shifted towards biological materials.5 There is ever increasing interest in exploration of natural polymers, and in particular polysaccharides, to generate efficient and environmentally friendly catalysts. Polysaccharides have many structural advantages viz. abundance, suitable functionality for the chelation of metallic species, numerous stereo-centres and chemical stability with a biodegradable nature.6 Recently polysaccharides like starch, cellulose and chitosan are being used as solid supports in heterogeneous catalytic systems.7 The presence of readily functionalizable amino groups and insolubility in most of the organic solvents and water favours chitosan over chitin for use in heterogeneous catalysis.8 A copolymer of β(1 → 4)-2-amino-deoxy-D-glucopyranose and 2-acetamido-2-deoxy-D-glucopyranose, chitosan is prepared by deacetylation of chitin, which is the second most abundant biopolymer after cellulose (Scheme 1).9
Chitosan has excellent stabilization strength for metal nanoparticles due to the bi-functionality of hydroxyl and amine groups in its molecular structure. This polymer has shown potential in the field of catalysis. One of the authors recently reported the copper mediated conversions of 3-carboxy-4-quinolones into 3-nitro-4-quinolones via ipso-nitration.10 As a high stoichiometric ratio of copper was required in the previous study, the authors focused on immobilizing the copper on polysaccharides and studied the effect of the operability of the reaction in the synthesis of 3-nitro-4-quinolones. The ipso nitration especially decarboxylative nitration appeared to be a significant synthetic approach in the synthesis of nitro compounds with high regioselectivity.11 Recently, Konwar and co-workers reported the cellulose supported Cu-NP (copper nanoparticle) catalysed decarboxylative nitration of aromatic α,β-unsaturated carboxylic acids.12 The scope of the reaction was limited to aromatic α,β-unsaturated carboxylic acids. Nevertheless, to the best of our knowledge, no work on chitosan-Cu-NP catalyst for the decarboxylative nitration of 3-carboxy-4-quinolones has been reported. The synthesis of 3-nitro quinolones was our prime concern as these molecules are less explored as compared to other quinolone derivatives. Hai-Hong Li et al. have also shown that 3-nitro quinolones are a potent inhibitor of EGFR (epidermal growth factor receptor).13 Other 3-nitro quinolone derivatives were also screened for their antihistaminic, antiprotozoal and antimalarial activities.14 Recently one of the authors explored the potential of 3-nitroquinole as an inhibitor of Brugia malayi thymidylate kinase.10 In the continuation of developing novel methodologies for the synthesis of biologically important scaffolds, the synthesized nitro derivatives were further functionalized and screened for their antibacterial activity.10,15 Hence a mild, cost effective and environmentally benign method for the synthesis of 3-nitro quinolones has been developed using chitosan-Cu-NP and its efficacy further equated with cellulose-Cu-NP and starch-Cu-NP. The results of this study are reported in this manuscript.
The required 4-quinolone-3-carboxylic acids were synthesized according to the reported method,16 starting from 3-chloro-4-fluoro aniline (2) and diethyl-2-(ethoxymethylene) malonate (EMME) (3), which on condensation followed by thermal cyclization in diphenyl ether yielded ester (5) via 4. The N alkylation of 5 with alkyl halide by K2CO3 in DMF and NaH in DMF (for cyclo-prop) yielded the N-alkylated derivatives (6). The N-alkylated derivatives were then treated with 2 N HCl to afford acids 7 by hydrolysis (Scheme 2). After getting the 4-quinolone-3-carboxylic acids the experiment began with the intention of optimizing the reaction parameters for decarboxylative nitration.
 | ||
| Scheme 2 Synthetic route for the synthesis of 4-quinolone-3-carboxylic acids by chit-Cu-NP and NO2BF4. | ||
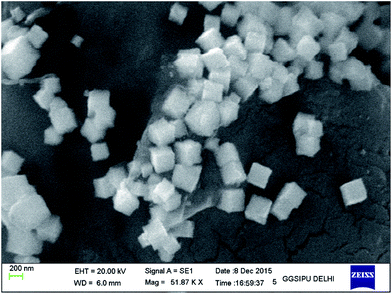
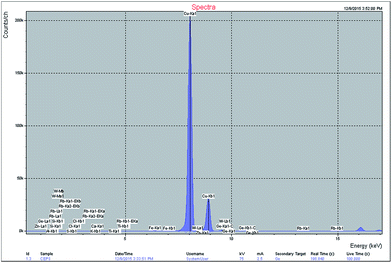
The polysac-Cu-NPs were synthesized by the chemical reduction method17 and characterization was done using X-ray diffraction (XRD), energy dispersive X-ray fluorescence (EDXRF), Scanning Electron Microscopy (SEM) and IR analysis. As shown in Fig. 1, the nanoparticles exhibited a highly crystalline nature. The diffraction peaks at 2θ = 43.5, 50.6, and 74.3 can be indexed as the [111], [200] and [220] planes of copper with face centered cubic (fcc) symmetry. The spectrograph showed no sign of impurities like CuO and Cu2O. The results are in good agreement with those reported for Cu-NP prepared by different methods.18 To determine the morphology of the prepared chitosan supported copper catalyst, SEM study at an accelerating voltage of 20 kV was employed. The SEM images of the catalyst taken before and after five times of use in the reaction (Fig. 2 and 3) show no noteworthy change in the morphology indicating extensive retention of the catalytic activity after recycling five times. The size of the nanoparticles was found to be in the order of 266 nm. The EDXRF analysis confirmed the presence of copper in the synthesised heterogeneous catalyst at 8.682% composition (Fig. 4). The IR analysis was steered to determine the molecular interaction(s) between the chitosan and the synthesized NPs.19
 | ||
| Fig. 1 XRD pattern of the chit-Cu-NP synthesized (red) and chit-Cu-NP after the fifth cycle (black). | ||
At first, an assessment of the reaction parameters, including, polysac-Cu-NP, nitrating agent, and solvent, was conducted at a fixed temperature (100 °C) and reaction time (10 h), using 7-chloro-1-ethyl-6-fluoro-4-quinolone-3-carboxylic acid (7a) as a model. Initially, the significance of the polysaccharide support was ascertained using chitosan-Cu-NP, cellulose-Cu-NP and starch-Cu-NP. In the first trial AgNO3 was used as a nitration agent and water as a solvent. With chit-Cu-NP (10 mol%) 62% nitro derivative was formed within 8 h, on the other hand cell-Cu-NP and starch-Cu-NP were found to be less effective as they yielded the desired nitro derivative in <55% yield in 10 h with the recovery of substrate 7a (Table 1 entries 1–3). The 20 mol% chit-Cu-NP was found to be essential for an efficient conversion producing 8a in 77% yield within 10 h (Table 1, entries 4–6). The high stoichiometric ratio of chit-Cu-NP (30 mol%) does not bear any effect on the yield (Table 1, entry 7). After gaining product in 77% yield further screening with other metal nitrates was carried out. The mono-nitrating agents NaNO3 and KNO3 produced 8a in 23 and 33% yield respectively (Table 1, entries 8–9). However, the use of di-nitrates like Ni, Co, Zn, Ca, Cu, and Pb yields the product in <63% (Table 1, entries 10–15). The tri-nitrates were found to be much more effective than any other nitrates used as Fe and Bi nitrates resulted in the decarboxylative nitration in 71 and 78% yield respectively. The nitrates used generated NO2+ in the reaction medium, which is responsible for the nitration. Therefore it was considered of interest that if nitronium ion was directly added to the reaction medium it may lead to an increase in the yield. The nitronium salts are excellently explored in organic synthesis and among all of them nitronium tetrafluoroborate (NO2BF4) is the most important one.20 NO2BF4 is a crystalline solid, which in the presence of water decomposes into HF and HNO3, so while using NO2BF4 (1 equivalent) the reaction was screened in acetonitrile at reflux temperature which resulted in the formation of 8a in 84% yield. The required stoichiometric quantity was also screened for NO2BF4 and it was found that 1.2 equivalents yielded 88% of 8a and higher equivalents did not have any effect on the yield. The catalyst loading was also screened in the case of NO2BF4 and 5 mol% was found to be essential for the effective transformation (88% yield, Table 1, entries 18–20). It is notable that NO2BF4 was operative only in the presence of chit-Cu-NP (Table 1, entry 21). Evidently, both NO2BF4 and chit-Cu-NP are important for the reaction.
| Entry | Cat. | Mol% | MNO3b | Yieldc (%) |
|---|---|---|---|---|
| a Reaction conditions: 3-carboxy-4-quinolone (7a) (1 mmol), Cu-NP, nitrate (1.2 mmol), in water (5 ml mmol−1 with respect to 7a) at 100 °C.b Nitrates used in hydrated forms.c Isolated yield.d Reaction performed in ACN at reflux temperature.e Starting material recovered. | ||||
| 1 | Cell-Cu | 10 | AgNO3 | 48 |
| 2 | Starch-Cu | 10 | AgNO3 | 55 |
| 3 | Chit-Cu | 10 | AgNO3 | 62 |
| 4 | Chit-Cu | 5 | AgNO3 | 33 |
| 5 | Chit-Cu | 15 | AgNO3 | 69 |
| 6 | Chit-Cu | 20 | AgNO3 | 77 |
| 7 | Chit-Cu | 30 | AgNO3 | 76 |
| 8 | Chit-Cu | 20 | NaNO3 | 23 |
| 9 | Chit-Cu | 20 | KNO3 | 35 |
| 10 | Chit-Cu | 20 | Ni(NO3)2 | 63 |
| 11 | Chit-Cu | 20 | Co(NO3)2 | 27 |
| 12 | Chit-Cu | 20 | Zn(NO3)2 | 12 |
| 13 | Chit-Cu | 20 | Ca(NO3)2 | 55 |
| 14 | Chit-Cu | 20 | Cu(NO3)2 | 59 |
| 15 | Chit-Cu | 20 | Pb(NO3)2 | 62 |
| 16 | Chit-Cu | 20 | Fe(NO3)3 | 71 |
| 17 | Chit-Cu | 20 | Bi(NO3)3 | 78 |
| 18 | Chit-Cu | 20 | NO2BF4 | 84d |
| 19 | Chit-Cu | 10 | NO2BF4 | 87d |
| 20 | Chit-Cu | 5 | NO2BF4 | 88d |
| 21 | — | — | NO2BF4 | —e |
After producing 8a in 88% yield the reaction was also screened in different solvents. The NO2BF4 was used as a nitrating agent and due to its highly polar nature, it was considered of interest to use anhydrous polar solvents. Among the different solvents used DMF was found to facilitate the formation of 8a in an excellent yield of 92% (Table 2, entry 1).
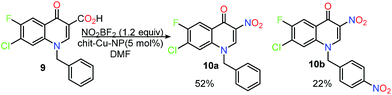
These findings steered us to explore the scope and limitations of this newly developed methodology. Therefore various therapeutically significant di-halo quinolones containing assorted alkyl groups at N-1, were selected for the nitration under optimised reaction conditions to give the corresponding 3-nitro derivatives. The results are summarised in Table 3. All the reactions gave excellent yields within a reaction time of 8–14 h. In order to widen the scope of this reaction the nitration of N1 benzylated quinolone 9 was carried out to see the effect of nitration on the aromatic ring of the alkyl aryl (benzyl) and aryl (3-chloro-4-fluoro-phenyl) groups. The 3-nitro derivative 10a was formed in 52% yield and some over nitrated product 10b especially on the benzylic part was noticed as observed by NMR and mass spectrometry (Scheme 3). The 3-nitro derivative was then successfully isolated by column chromatography using 10% CH3OH/CHCl3 as eluent.
| Entry | R | R′, R′′ | Yield (%) | Time (h) |
|---|---|---|---|---|
| 7a | Et | F/Cl | 92 | 10 |
| 7b | Isoprop | F/Cl | 85 | 8 |
| 7c | n-Butyl | F/Cl | 82 | 9 |
| 7d | sec-Butyl | F/Cl | 85 | 12 |
| 7e | Me | F/Cl | 79 | 10 |
| 7f | cyclo-Prop | F/Cl | 76 | 8 |
| 7g | n-Propyl | F/F | 86 | 12 |
| 7h | sec-Butyl | F/F | 88 | 14 |
| 7i | n-Pentyl | F/F | 91 | 12 |
| 7j | n-Octyl | F/F | 82 | 12 |
| 7k | Et | Cl/Cl | 79 | 10 |
| 7l | cyclo-Prop | Cl/Cl | 75 | 7 |
| 7m | n-Butyl | Cl/Cl | 89 | 9 |
| 7n | sec-Butyl | Cl/Cl | 79 | 8 |
| 7o | Heptyl | Cl/Cl | 89 | 10 |
After completion of the decarboxylative nitration reaction the reaction mixture was dried under vacuum and extracted with ethyl acetate after addition of water. The catalyst recovered after the reaction (blue coloured) was further activated by NaBH4 reduction (black coloured), and was stored under nitrogen atmosphere and could be reused for up to five cycles with negligible loss of activity towards the ipso nitration (Fig. 5 and 6). The recyclability and nature (deformation, if present) of the catalyst was further confirmed by SEM, XRD, EDXRF and ipso nitration of 3-caboxy-quinolone (Fig. 1 and 3). The EDXRF analysis confirmed the decrease in the percentage of copper in the catalyst after the fifth cycle (6.865%).
 | ||
| Fig. 5 (a) Recovered chit-Cu-NP after reaction (blue) and (b) chit-Cu-NP after regeneration by reduction (black). | ||
A plausible reaction mechanism was suggested for the copper-NP-mediated chelation of β-keto acid assisted decarboxylative nitration of the quinolones (Scheme 4). Initially the Cu oxidized to Cu2+ in the presence of atmospheric oxygen. The two carbonyl groups of the quinolones chelated through oxygen with Cu2+ ions (7′). The two carbonyl groups of the quinolones chelated through oxygen with Cu2+ ions. The decarboxylative nitration takes place through a concerted mechanism, in which NO2+ from nitronium tetrafluoroborate attacks at the charge developed on the C-3 carbon and decarboxylation takes place simultaneously, leading to formation of the product 8. The blue coloured recovered catalyst confirmed the presence of oxidised copper.
Biological evaluation
In the designing of new antimicrobial agents with reduced side effects and enhanced activity, lead optimization is one of the most important steps. In this process the molecule with an optimum pharmacological profile cannot be tuned or tailored because there is a chance of loss of desired biological activity. The major concerns are insufficient oral bioavailability due to lower solubility, metabolic instability, toxicity caused by reactive functional groups, and nonselective binding. Bioisosteric modification represents a significant approach for refining the pharmacological profiles of lead candidates and involves replacing undesirable structural features with bioisosteric groups or molecular structures which are known to display the same biological activities. Bioisosteres are not exact structural mimetics and are often more similar in biological rather than physical properties.21 Hereby the authors design novel 3-tetrazolyl bioisosteres (Fig. 7) of 4-quinolone-3-carboxylic acids and the synthesis was successfully achieved by the developed methodology. All of the synthesised compounds were screened for their antibacterial properties.The C-7 position of 8 was then substituted by piperazine in DMSO at 120–130 °C to get 11 which on reduction with anhydrous SnCl2 in HCl afforded 4-quinolone-3-amine (12). The amine (12) on treatment with triethyl orthoformate and sodium azide in acetic acid yielded the target molecule (13) in good to excellent yield. The non-piperazyl derivative (14) was also synthesized from the 3-nitro derivative (8) using the same sequence of reactions which were applied for synthesis of (13) (Scheme 5). In the present manuscript the lower alkyl derivatives were used for the synthesis of targeted molecules due to their importance in the mode of action of quinolones as antibacterial agents.22 The reaction was monitored by TLC, and after completion of the reaction, the compounds were isolated and purified by column chromatography. These compounds were characterized by their IR, NMR and mass spectra.
The evaluation of the antibacterial activity of compounds (13a–d) and (14a–d) against various strains of bacteria, for example, Staphylococcus aureus (ATCC 6538), Staphylococcus aureus (ATCC 25632) (MRSA), Escherichia coli (ATCC 49416), Salmonella typhii (ATCC 49416) and Vibrio cholera (ATCC 55868), was carried out according to a micro-dilution method (using a microtitre ELISA plate). These strains were chosen based on their clinical and pharmacological importance.23 Bacteria such as Staphylococcus have emerged with resistance to six and more different antibiotics.24 The minimum inhibitory concentration (MIC) of each compound was determined against test isolates using this technique. The MICs of the standard antibacterial drug ciprofloxacin were determined using Mueller–Hinton broth. The antibacterial activity was compared with ciprofloxacin which was used as a positive control. In all tests, the MIC values are expressed in μM. The compounds 14a–d had the same activity when compared with ciprofloxacin although these molecules lack the important piperazyl substitution at the C-7 position, which clearly reveals that the designed molecule with 3-tetrazolyl substitution plays an important role in the inhibition of gram positive as well as gram negative bacteria. The piperazine substitution at the C-7 position increases the activity as expected as compared to the standard drug ciprofloxacin. The pre-eminent inhibition shown by the compounds 13a–d against gram positive bacteria S. aureus and methicillin resistant S. aureus (MRSA) was from 12.5 to 25 μM. The norfloxacin and ciprofloxacin derivatives 13b and 13c showed activity at 12.5 and 25 μM respectively against bacterium S. typhi , while corresponding drugs are active at 100 μM. The designed molecules 13a–d also showed excellent activity against bacteria V. chloera and E. coli (Table 4). The solubilities of the synthesized compounds are promising as compared to the parent drug which may enhance the activity of the compounds. In the present manuscript it is evidenced that tertrazolyl substitution is a remarkably good bioisostere of carboxylic acid which increased the activity of the corresponding drug molecule.
| Sample | S. aureus | S. aureus (MRSA) | E. coli | S. typhi | V. cholerae |
|---|---|---|---|---|---|
| CSA001 (14a) | 100 | 100 | 100 | 100 | 50 |
| CSA003 (14b) | 100+ | 100+ | 100+ | 100+ | 100+ |
| CSA004 (14c) | 100 | 100 | 100 | 100 | 100 |
| CSA006 (14d) | 100+ | 100+ | 100+ | 100+ | 100+ |
| CSA002 (13a) | 12.5 | 25 | 12.5 | 25 | 12.5 |
| CSA005 (13b) | 12.5 | 12.5 | 12.5 | 25 | 12.5 |
| CSA007 (13c) | 12.5 | 12.5 | 25 | 12.5 | 25 |
| CSA008 (13d) | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 |
| Ciprofloxacin | 100 | 100 | 100+ | 100 | 100 |
Docking simulation
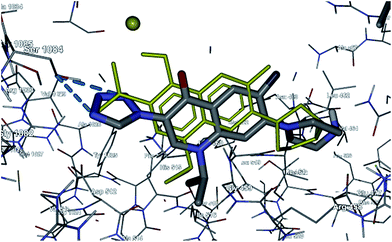
In order to explain the biological activity and SAR of the synthesized molecules, docking studies were carried out using Molegro Virtual Docker (MVD) on a windows based PC.25 The direct evidence regarding the interaction of quinolones with DNA gyrase or DNA was accessible after the discovery of the co-crystal structure of the DNA gyrase with ciprofloxacin which provided insights about the inhibitory function of quinolones. The reported co-crystallized structure of the protein in complex with ciprofloxacin (pdb id 2XCT) with 3.35 Å resolution was used for the current docking studies.26 The docking protocol of MVD was validated by predicting the binding mode of the crystallographic ligand ciprofloxacin. The protein–ligand complex obtained from the docking simulation revealed that the docked ciprofloxacin was nearly superimposed on the native co-crystallized one (yellow). Fig. 8 noticeably shows that MVD successfully predicted the binding mode of the crystallographic ligand with a root-mean square (RMS) deviation of 0.521 Å. The hydrogen bond interactions between the protein amino acids and both the native X-ray ciprofloxacin and docked ciprofloxacin were the same (Fig. 8). The docked ciprofloxacin forms a coordination bond with the manganese ion via the carbonyl and carboxylate group’s oxygens with 1.8 Å and 2.1 Å distance respectively while the co-crystallized ciprofloxacin forms a bond with manganese of 2.1 Å with its carbonyl and carboxylate groups. The carboxylate group of the docked ciprofloxacin forms a hydrogen bond with the –OH of Ser1084 and the nitrogen atom of the piperazine ring performs hydrogen bonding with the side chain carboxylate group of Glu477.Compound 13d which is the most active compound showed a very similar binding mode compared to the co-crystallized ciprofloxacin (Fig. 9). The tetrazole formed three hydrogen bonds with Ser1084 instead of one which is formed by the native co-crystallized ciprofloxacin. The bond lengths of the three hydrogen bonds were 1.9, 2.1 and 3.1 Å. The nitrogen of the piperazine formed a hydrogen bond with base pair DNA backbone DT4. The MolDock score (kcal mol−1) and the Rerank Score was −123.54 and −74.67 respectively for 13d. Overall, compound 13d docked in the binding site of the enzyme in a similar manner to ciprofloxacin with additional hydrogen bonds related to the 3-tetrazole scaffold, supporting the molecular design of the compound A prototype (Fig. 7).
Conclusions
To conclude, through a one-step reduction in aq. NaBH4, chitosan supported Cu nanoparticles were prepared and characterized by state of the art techniques. The synthesized heterogeneous catalysts led to decarboxylative nitration of 3-carboxy-4-quinolone in a single step. The chit-Cu-NP can be recycled for catalytic purposes which is confirmed for up to five cycles. The synthesized 3-nitro derivatives successfully transformed into the 3-tetrazolyl bioisosteres possessing high antimicrobial activity without the significant loss of activity of the corresponding drugs. This noticeable in vitro efficacy of these compounds against gram positive as well as gram negative bacteria is indicative of the potential utility of these compounds in the therapy of bacterial infection and methicillin resistant S. aureus infection.Acknowledgements
CSA acknowledges GGS Indraprastha University, New Delhi for financial assistance and TRC Lab for providing analytical data. We are also thankful to BioGenics, Karnataka for providing antibacterial activity on payment basis.References
- (a) P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC; (b) C.-J. Li and B. M. Trost, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 13197–13202 CrossRef CAS PubMed.
- (a) R. A. Sheldon and H. Van Bekkum, Fine chemicals through heterogeneous catalysis, John Wiley & Sons, 2008 Search PubMed; (b) J. M. Thomas and W. J. Thomas, Principles and practice of heterogeneous catalysis, John Wiley & Sons, 2014 Search PubMed; (c) D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852–7872 CrossRef CAS PubMed; (d) N. Mizuno and M. Misono, Chem. Rev., 1998, 98, 199–218 CrossRef CAS PubMed.
- (a) J. Lu and P. H. Toy, Chem. Rev., 2009, 109, 815–838 CrossRef CAS PubMed; (b) J. H. Clark and C. N. Rhodes, Clean synthesis using porous inorganic solid catalysts and supported reagents, Royal Society of Chemistry, 2000 Search PubMed.
- (a) X.-Z. Shu, S. C. Nguyen, Y. He, F. Oba, Q. Zhang, C. Canlas, G. A. Somorjai, A. P. Alivisatos and F. D. Toste, J. Am. Chem. Soc., 2015, 137, 7083–7086 CrossRef CAS PubMed; (b) G. Zhu, C. Wang, Y. Zhang, N. Guo, Y. Zhao, R. Wang, S. Qiu, Y. Wei and R. H. Baughman, Chem.–Eur. J., 2004, 10, 4750–4754 CrossRef CAS PubMed; (c) J. H. Clark, M. G. Dekamin and F. M. Moghaddam, Green Chem., 2002, 4, 366–368 RSC; (d) G. Zhu, C. Wang, Y. Zhang, N. Guo, Y. Zhao, R. Wang, S. Qiu, Y. Wei and R. H. Baughman, Chem.–Eur. J., 2004, 10, 4750–4754 CrossRef CAS PubMed; (e) C. Petrucci, M. Cappelletti, O. Piermatti, M. Nocchetti, M. Pica, F. Pizzo and L. Vaccaro, J. Mol. Catal. A: Chem., 2015, 401, 27–34 CrossRef CAS; (f) A. Dhakshinamoorthy and H. Garcia, Chem. Soc. Rev., 2012, 41, 5262–5284 RSC; (g) I. X. Green, W. Tang, M. Neurock and J. T. Yates, Science, 2011, 333, 736–739 CrossRef CAS PubMed; (h) D. Tsukamoto, Y. Shiraishi, Y. Sugano, S. Ichikawa, S. Tanaka and T. Hirai, J. Am. Chem. Soc., 2012, 134, 6309–6315 CrossRef CAS PubMed; (i) M. Pietrowski, M. Zieliński and M. Wojciechowska, ChemCatChem, 2011, 3, 835–838 CrossRef CAS; (j) M. Zieliński and M. Wojciechowska, Catal. Commun., 2012, 18, 1–4 CrossRef; (k) D. Azarifar and F. Soleimanei, RSC Adv., 2014, 4, 12119–12126 RSC; (l) B. H. Lipshutz, D. M. Nihan, E. Vinogradova, B. R. Taft and Z. V. Boskovic, Org. Lett., 2008, 10, 4279–4282 CrossRef CAS PubMed; (m) B. H. Lipshutz, J. B. Unger and B. R. Taft, Org. Lett., 2007, 9, 1089–1092 CrossRef CAS PubMed; (n) M. B. Gawande, Y. Monga, R. Zboril and R. Sharma, Coord. Chem. Rev., 2015, 288, 118–143 CrossRef CAS; (o) R. N. Baig, M. N. Nadagouda and R. S. Varma, Coord. Chem. Rev., 2015, 287, 137–156 CrossRef.
- (a) M. G. Dekamin, M. Azimoshan and L. Ramezani, Green Chem., 2013, 15, 811–820 RSC; (b) A. Primo, T. Marino, A. Corma, R. Molinari and H. Garcia, J. Am. Chem. Soc., 2011, 133, 6930–6933 CrossRef CAS PubMed; (c) J. Sun, J. Wang, W. Cheng, J. Zhang, X. Li, S. Zhang and Y. She, Green Chem., 2012, 14, 654–660 RSC.
- (a) S. Dumitriu, in Polysaccharides: Structural Diversity and Functional Versatility, Marcel Dekker, New York, 2005 Search PubMed; (b) M. Yalpani, Polysaccharides: syntheses, modifications and structure/property relations, Elsevier, 2013 Search PubMed.
- (a) A. Khalafi-Nezhad and F. Panahi, Green Chem., 2011, 13, 2408–2415 RSC; (b) A. Khalafi-Nezhad and F. Panahi, J. Organomet. Chem., 2012, 717, 141–146 CrossRef CAS; (c) D. Baruah, R. N. Das, S. Hazarika and D. Konwar, Catal. Commun., 2015, 72, 73–80 CrossRef CAS; (d) B. Jia, Y. Mei, L. Cheng, J. Zhou and L. Zhang, ACS Appl. Mater. Interfaces, 2012, 4, 2897–2902 CrossRef CAS PubMed; (e) P. Cotugno, M. Casiello, A. Nacci, P. Mastrorilli, M. M. Dell’Anna and A. Monopoli, J. Organomet. Chem., 2014, 752, 1–5 CrossRef CAS.
- (a) R. N. Baig and R. S. Varma, Green Chem., 2013, 15, 1839–1843 RSC; (b) R. N. Baig, M. N. Nadagouda and R. S. Varma, Green Chem., 2014, 16, 2122–2127 RSC; (c) Y. Qin, W. Zhao, L. Yang, X. Zhang and Y. Cui, Chirality, 2012, 24, 640 CrossRef CAS PubMed.
- (a) R. A. Muzzarelli, Chitin, Elsevier, 2013 Search PubMed; (b) J. Zikakis, Chitin, chitosan, and related enzymes, Elsevier, 2012 Search PubMed.
- C. S. Azad, V. M. Balaramnavar, I. A. Khan, P. K. Doharey, J. K. Saxena and A. K. Saxena, RSC Adv., 2015, 5, 82208–82214 RSC.
- (a) G. Prakash and T. Mathew, Angew. Chem., Int. Ed., 2010, 49, 1726–1728 CrossRef CAS PubMed; (b) S. Manna, S. Jana, T. Saboo, A. Maji and D. Maiti, Chem. Commun., 2013, 49, 5286–5288 RSC; (c) K. Ramesh, S. Shylaja, K. Rajanna, P. Giridhar Reddy and P. Saiprakash, Adv. Phys. Chem., 2013, 146585, DOI:10.1155/2013/146585; (d) S. Maity, T. Naveen, U. Sharma and D. Maiti, Org. Lett., 2013, 15, 3384–3387 CrossRef CAS PubMed; (e) T. Naveen, S. Maity, U. Sharma and D. Maiti, J. Org. Chem., 2013, 78, 5949–5954 CrossRef CAS PubMed; (f) B. V. Rokade and K. R. Prabhu, J. Org. Chem., 2014, 79, 8110–8117 CrossRef CAS PubMed.
- D. Baruah, P. Pahari and D. Konwar, Tetrahedron Lett., 2015, 56, 2418–2421 CrossRef CAS.
- H. H. Li, H. Huang, X. H. Zhang, X. M. Luo, L. P. Lin, H. L. Jiang, J. Ding, K. Chen and H. Liu, Acta Pharmacol. Sin., 2008, 29, 1529 CrossRef CAS PubMed.
- (a) R. G. Glushkov and N. K. Davydova, Khim.-Farm. Zh., 1992, 26, 43 CAS; (b) N. K. Davydova, N. B. Marchenko, R. G. Glushkov, V. V. Peters and E. N. Padeiskaya, Pharm. Chem. J., 1991, 25, 730 CrossRef; (c) A. R. Surrey and R. A. Cutler, J. Am. Chem. Soc., 1951, 73, 2413 CrossRef CAS.
- (a) C. S. Azad and A. K. Saxena, Org. Chem. Front., 2015, 2, 665–669 RSC; (b) C. S. Azad, S. S. Bhunia, A. Krishna, P. K. Shukla and A. K. Saxena, Chem. Biol. Drug Des., 2015, 86, 440–446 CrossRef CAS PubMed; (c) C. S. Azad and A. K. Saxena, Tetrahedron, 2013, 69, 2608–2612 CrossRef CAS; (d) C. S. Azad and A. K. Narula, RSC Adv., 2015, 5, 100223–100227 RSC.
- H. Koga, A. Itoh, S. Murayama, S. Suzue and T. Irikura, J. Med. Chem., 1980, 23, 1358 CrossRef CAS PubMed.
- X. Cheng, X. Zhang, H. Yin, A. Wang and Y. Xu, Appl. Surf. Sci., 2006, 253, 2727–2732 CrossRef CAS.
- (a) D. Mott, J. Galkowski, L. Wang, J. Luo and C.-J. Zhong, Langmuir, 2007, 23, 5740–5745 CrossRef CAS PubMed; (b) S. Panigrahi, S. Kundu, S. K. Ghosh, S. Nath, S. Praharaj, S. Basu and T. Pal, Polyhedron, 2006, 25, 1263–1269 CrossRef CAS.
- M. S. Usman, N. A. Ibrahim, K. Shameli, N. Zainuddin and W. M. Z. W. Yunus, Molecules, 2012, 17, 14928–14936 CrossRef CAS PubMed.
- (a) P. Natarajan, R. Chaudhary and P. Venugopalan, J. Org. Chem., 2015, 80, 10498–10504 CrossRef CAS PubMed; (b) G. A. Olah, G. K. S. Prakash, Q. Wang and X.-Y. Li, in Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, Ltd, 2001, DOI:10.1002/047084289X.rn043.
- D. J. Wood, J. D. Vlieg, M. Wagener and T. Ritschel, J. Chem. Inf. Model., 2012, 52, 2031–2043 CrossRef CAS PubMed.
- Y. Asahina, T. Ishizaki and S. Suzue, Prog. Drug Res., 1992, 38, 57–106 CAS.
- A. W. McCracken and R. A. Cawson, Clinical and oral microbiology, Hemisphere Publishing Corporation, Washington, DC (USA), 1983, p. 512 Search PubMed.
- F. D. Lowy, J. Clin. Invest., 2003, 111, 1265 CrossRef CAS PubMed.
- R. Thomsen and M. H. Christensen, J. Med. Chem., 2006, 49, 3315–3321 CrossRef CAS PubMed.
- B. D. Bax, P. F. Chan, D. S. Eggleston, A. Fosberry, D. R. Gentry, F. Gorrec, I. Giordano, M. M. Hann, A. Hennessy and M. Hibbs, Nature, 2010, 466, 935–940 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C spectra of compounds, detail of EDRXF and other SEM images. See DOI: 10.1039/c5ra26909a |
| This journal is © The Royal Society of Chemistry 2016 |