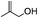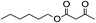Ag–Cu nanoparticles as efficient catalysts for transesterification of β-keto esters under acid/base-free conditions†
Hongmei Yueab,
Hao Yuab,
Sheng Liuab and
Chunli Xu*ab
aKey Laboratory of Applied Surface and Colloid Chemistry (Shaanxi Normal University), Ministry of Education, Xi'an 710119, PR China. E-mail: xuchunli@snnu.edu.cn; Tel: +86-29-81530779
bSchool of Chemistry and Chemical Engineering, Shaanxi Normal University, Chang'an West Street 620, Xi'an 710119, PR China
First published on 9th February 2016
Abstract
Transesterification of β-keto esters and alcohols are traditionally catalyzed by acid or basic catalysts. However, these traditional catalysts do not always meet the requirements of modern synthetic chemistry which need to be highly efficient, selective, and environmentally friendly. In this work, Ag–Cu metal sites were first introduced as transesterification catalysts. The effect of the support, Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu molar ratio, and reaction conditions were investigated. The Ag–Cu metal sites were proved to be active in the β-ketoester transesterification with various alcohols, having yields comparable to the conventional acid- or base-catalysts.
Cu molar ratio, and reaction conditions were investigated. The Ag–Cu metal sites were proved to be active in the β-ketoester transesterification with various alcohols, having yields comparable to the conventional acid- or base-catalysts.
1. Introduction
β-Keto esters have been given much attention because of their broad use, especially in the organic synthesis of a number of complex biologically active natural products of pharmaceuticals, chemicals and polymers.1 Several synthetic methods have been reported on the preparation of these β-keto esters, including Claisen condensation,2 carbonylation of aryl halides,3 acylation of Meldrum's acid,4 and transesterification.5 In this regard, the transesterification reaction of β-ketoesters has been recognized as one of the most significant processes in producing other β-ketoesters,6 because it allows for the preparation of more complex products from more easily accessible synthons (Scheme 1).7Transesterification is the process of exchanging the organic group R2 of an ester with the organic group R1 of an alcohol (Scheme 1). It is an equilibrium-driven process.8 It has long been known that the reaction is accelerated by acid or base catalysts.9 Because of their versatility, acid- or base-catalyzed reactions were the subjects of extensive investigation. A variety of acid or basic catalysts have been reported for transesterification of β-ketoesters in the literature, such as homogeneous Brönsted acids and bases (hydrochloric acids,10 sulfuric acids, phosphoric acids, sodium alkoxides, potassium alkoxides,11 and cesium carbonate12), homogeneous Lewis acids and bases (ZnCl2, FeCl3·6H2O,10 LiClO4,13 InI3,14 NH2SO3H,15 yttria–zirconia,16 sodium perborate,17 and KCN11), solid acid catalysts (montmorillonite K-10,18 SG–NH2–Bpy–Zn,19 Mo–ZrO2,20 sulphated SnO2,21 B2O3/ZrO2,22 and minerals clay23), solid basic catalysts (zinc(II) oxide,24 Mg–Al–O–t-butyl hydrotalcite,25 and basic silica26). Homogeneous acid/basic catalysts are generally corrosive to equipment and difficult to recycle, giving rise to serious environmental and corrosion problems. The use of heterogeneous acid/basic catalysts simplifies greatly the technological process by facilitating the separation of the post-reaction. Unfortunately, the heterogeneous acid/basic catalysts usually showed low activity.11 One kind of substitute to acid/basic catalysts are enzymes. However, enzymes are easily deactivated. Therefore, the attention has to be diverted to discover new catalysts.27
In the transesterification mechanism, the carbonyl carbon of the starting ester (RCOOR2) undergoes nucleophilic attack by the incoming alkoxide (R1O−) to give a tetrahedral intermediate, which proceeds to the transesterified product (RCOOR1). Alkoxide (R1O−) is generated by removing a proton from the starting alcohol (R1OH). It can be accelerated by activating the O–H bond in the alcohol on the surface of catalyst. Since the forming rate of alkoxide (R1O−) decides the reaction rate of transesterification,28 it is essential to find an efficient catalyst which have the ability to activate the O–H bond in the alcohol. Metal catalysts, such as Au, Pt, Pd, Ni, Cu, and Ag, are one important type of catalysts in chemistry and chemical engineering.29–31 The metal sites could activate the O–H bond in alcohol.32 In other sides, the metal catalysts have vacant orbitals. The vacant orbitals of metals could coordinate to the oxygen of β-keto esters. The coordination increases the electrophilicity of the carbonyl carbon. The increase in electrophilicity of esters could also accelerate the rate of transesterification.33,34 Therefore, metal catalysts may offer the potential for catalyzing the reaction, the investigation of which is the focus of this work. Catalysts based on Cu or Ag have attracted much attention in heterogeneous catalysis because of the metal's relative cost advantage. Besides, bimetallic catalysts frequently exhibit better catalytic performance than their monometallic counterparts and have therefore attracted increasing attention from both academic and industrial fields.35 In this work, hydrotalcite-like materials supported Ag–Cu catalysts (Ag–Cu/HTs) were prepared and tested in the transesterification of β-ketoester and alcohols.
2. Experimental section
2.1. Catalyst preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+molar ratio of 5
Al3+molar ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and another aqueous solution (240 mL) containing NaOH (1.5 mol L−1) were slowly dropped into Na2CO3 (40 mL, 1.5 mol L−1) aqueous solution with continuous stirring at room temperature. Afterwards, a white suspension was gained and the pH value of mixture was controlled between 9 and 10 with the alkaline solution. Then, the suspension was aged at 60 °C for 18 h with constant stirring to generate the hydrotalcite-like structure. The resulting precipitate was filtered with the deionized water until the pH value of mixture was about 7 after the suspension has been cooled to room temperature. Finally mixture was dried at 110 °C for 12 h in air. The resulting material was Mg–Al hydrotalcite-like support. Finally, the support having a nominal molar ratio of Mg
1 and another aqueous solution (240 mL) containing NaOH (1.5 mol L−1) were slowly dropped into Na2CO3 (40 mL, 1.5 mol L−1) aqueous solution with continuous stirring at room temperature. Afterwards, a white suspension was gained and the pH value of mixture was controlled between 9 and 10 with the alkaline solution. Then, the suspension was aged at 60 °C for 18 h with constant stirring to generate the hydrotalcite-like structure. The resulting precipitate was filtered with the deionized water until the pH value of mixture was about 7 after the suspension has been cooled to room temperature. Finally mixture was dried at 110 °C for 12 h in air. The resulting material was Mg–Al hydrotalcite-like support. Finally, the support having a nominal molar ratio of Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al = 5
Al = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used to study the catalytic activity, unless otherwise noted.
1 was used to study the catalytic activity, unless otherwise noted.γ-Al2O3 was prepared by calcining γ-Al2O3 (a commercial sample, Alfa Aesar Chemicals Co., Ltd.) at 400 °C for 5 h in air. Similarly, MgO was prepared by calcining MgO sample (AR, 98.5%, Sinopharm Chemical Reagent Co., Ltd.) at 500 °C for 3 h in air.
The activated-carbon (AC) support was prepared by treating commercial charcoal active powder (AR, Tianli Chemical Reagent Co., Ltd.) with HNO3 (6 mol L−1, 60 mL) in a three-necked batch reactor fitted with a reflux condenser, oil bath and magnetic stirrer at 100 °C for 3 h. The slurry was then cooled to room temperature, filtered, and washed with deionized water until the pH value of filtrate was about 7. The carbon was dried at 120 °C for 3 h and then calcined at 300 °C for 3 h.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu molar ratio = 1
Cu molar ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 1.8
0, 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.2
1, 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.59
1, 0.59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.29
1, 0.29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, or 0
1, or 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with different Ag–Cu content (1 wt%, 2 wt%, 3 wt%, 4 wt%, 5 wt%, 7 wt%, 8 wt%) by a sol-immobilisation method.37 Cu(NO3)2·3H2O (AR, 99%, Sinopharm Chemical Reagent Co., Ltd) and AgNO3 (99%, Reagent No.1 Factory Of Shanghai Chemical Reagent Co., Ltd.) of the desired concentration were used as metal precursors and were added simultaneously, drop by drop, to a beaker containing deionized water to form an aqueous mixture. Then the poly(vinyl alcohol) (PVA) dispersion (1 mL, 1 mg mL−1) as the stabilizer was added (PVA/(Ag + Cu) (w/w) = 0.125) with vigorous stirring for 2 min. After that, NaBH4 prepared freshly (0.1 mol L−1 aqueous solution, NaBH4/metal mole fraction 0.24) was rapidly dropped into the mixture solution as the reducing agent. The dispersion was stirred for 30 min, and followed by the introduction of the desired support materials. After 2 h of stirring, the mixture was filtered, washed with distilled water and dried at 110 °C in air overnight. The resulted materials were Ag–Cu supported catalysts. Hereafter, if there is no other special statement, 5 wt% Ag–Cu/HTs (1.2
1) with different Ag–Cu content (1 wt%, 2 wt%, 3 wt%, 4 wt%, 5 wt%, 7 wt%, 8 wt%) by a sol-immobilisation method.37 Cu(NO3)2·3H2O (AR, 99%, Sinopharm Chemical Reagent Co., Ltd) and AgNO3 (99%, Reagent No.1 Factory Of Shanghai Chemical Reagent Co., Ltd.) of the desired concentration were used as metal precursors and were added simultaneously, drop by drop, to a beaker containing deionized water to form an aqueous mixture. Then the poly(vinyl alcohol) (PVA) dispersion (1 mL, 1 mg mL−1) as the stabilizer was added (PVA/(Ag + Cu) (w/w) = 0.125) with vigorous stirring for 2 min. After that, NaBH4 prepared freshly (0.1 mol L−1 aqueous solution, NaBH4/metal mole fraction 0.24) was rapidly dropped into the mixture solution as the reducing agent. The dispersion was stirred for 30 min, and followed by the introduction of the desired support materials. After 2 h of stirring, the mixture was filtered, washed with distilled water and dried at 110 °C in air overnight. The resulted materials were Ag–Cu supported catalysts. Hereafter, if there is no other special statement, 5 wt% Ag–Cu/HTs (1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of Ag/Cu molar ratio, and 5
1 of Ag/Cu molar ratio, and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of Mg
1 of Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio) was used.
Al molar ratio) was used.2.2. Characterization of supports and catalysts
Scanning transmission electron micrographs (STEM) were obtained using a FEI Tecnai G2 F20 instrument (USA).X-ray photoelectron spectroscopy (XPS) was used to characterize the elements valence present in the catalysts. XPS data was obtained in an Axis Ultra spectrometer (Kratos Analytical Ltd., Japan) with Al Kα X-ray source (1486.6 eV, 15 kV, 5 mA) at an energy of 150 W.
Elemental analysis of HTs was carried out by an FEI Quanta 200 (USA) scanning electron microscope equipped with energy-dispersive X-ray spectroscopy.
The loading amount of Ag–Cu was determined by inductively coupled plasma mass spectrometry (ICP-MS; M90, Bruker, Germany) and Atomic Absorption Spectroscopy (AAS; ZA3000, Hitachi, Japan).
X-ray diffraction analysis (XRD) was collected on X-ray diffractometer of Rigaku Co. Japan (D/Max2550VB+/PC) with Cu-Kα radiation at 40 kV and 450 mA.
Micromeritics ASAP 2020 instrument (America) was used to determine the surface area, average pore diameter, as well as pore distribution of Ag–Cu/HTs using Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) method. The sample was degassed at 110 °C for 4 h in N2 prior to carrying out surface area measurements.
2.3. General experimental procedure
In our experimental procedure, unless otherwise noted, all reagents were used as received without further purification. The reaction tests were performed in a 50 mL flask equipped with oil bath and magnetic stirrer at 110 °C for 6 h. The cinnamyl alcohol (11 mmol, 9 Dingchem Co., Ltd) and ethyl acetoacetate (11 mmol, Sinopharm Chemical Reagent Co., Ltd) were added into the flask with DMF (1.5 mL, Sinopharm Chemical Reagent Co., Ltd). After the completion of reaction, the mixture was cooled to room temperature and the catalyst was removed by centrifugation. The DMF solvent was removed by extraction. The yield was characterized by the 1H-NMR spectrum of the crude reaction mixture. The crude reaction mixture was further purified by column chromatography using hexane and ethyl acetate as eluents (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 10
ethyl acetate 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–5
1–5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on silica gel to obtain the pure products.
1) on silica gel to obtain the pure products.
3. Results and discussion
3.1. The characterization of catalysts
| Catalysts | Surface area (m2 g−1) | Pore volume (cm3 g−1) | Average pore diameter (Å) |
|---|---|---|---|
| HTs | 35 | 0.18 | 158 |
| Ag–Cu/HTs | 29 | 0.16 | 150 |
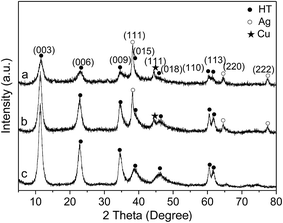
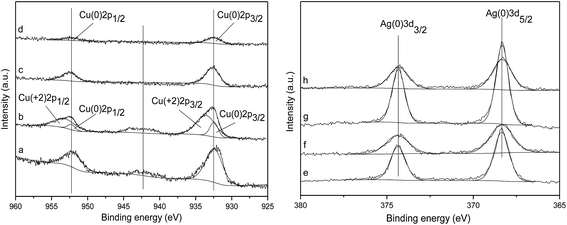
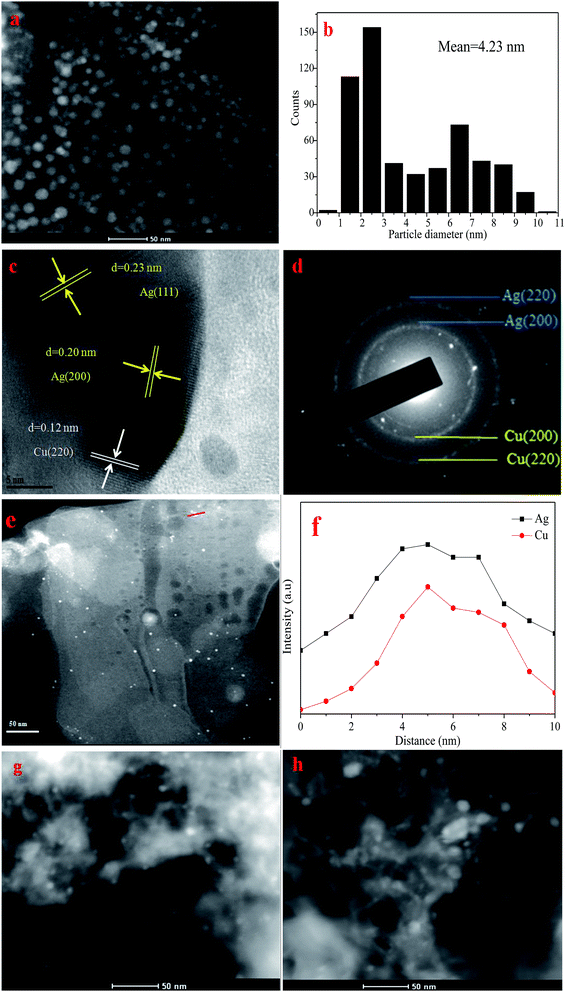
Energy-dispersive X-ray spectroscopy (EDS) analysis was also carried out. The variations of composition across the red line in STEM image (Fig. 3e) are present in Fig. 3f. The line scan shows that Ag and Cu were mixed well in the nanoparticles. Combined with the XPS and XRD analysis, it can be concluded that both Cu and Ag nanoparticles were in a metallic state and mixed well.
The structure of the used Ag–Cu/HTs was also determined. HR-TEM image reveals that the structural properties of Ag–Cu nanoparticle did not change after four times used (Fig. 3g). After six times used, a little part of Ag–Cu nanoparticle were found to be agglomerated (Fig. 3h).
3.2. The investigation of active sites
Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio may affect the acid/basic property of HTs.11,25 Because the acid/basic property of catalysts may affect the reaction rate of transesterification,11 the effect of Mg
Al molar ratio may affect the acid/basic property of HTs.11,25 Because the acid/basic property of catalysts may affect the reaction rate of transesterification,11 the effect of Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
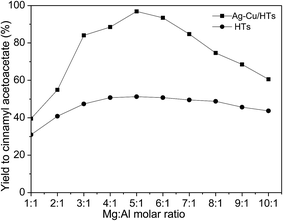
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio on neat HTs and Ag–Cu/HTs were studied. As shown in Fig. 4, the yield to cinnamyl acetoacetate changed with Mg
Al molar ratio on neat HTs and Ag–Cu/HTs were studied. As shown in Fig. 4, the yield to cinnamyl acetoacetate changed with Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio for both neat HTs and Ag–Cu/HTs. In the presence of neat HTs, the yield increased with Mg
Al molar ratio for both neat HTs and Ag–Cu/HTs. In the presence of neat HTs, the yield increased with Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio. At 4
Al molar ratio. At 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of Mg
1 of Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio, the yield reached the plateau of maximum. Above 6
Al molar ratio, the yield reached the plateau of maximum. Above 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the yield decreased with Mg
1, the yield decreased with Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio. In the presence of Ag–Cu/HTs, the yield also increased with Mg
Al molar ratio. In the presence of Ag–Cu/HTs, the yield also increased with Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio. At 5
Al molar ratio. At 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of Mg
1 of Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio, the yield reached the maximum. Above 5
Al molar ratio, the yield reached the maximum. Above 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the yield decreased with Mg
1, the yield decreased with Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio. This indicated that Mg
Al molar ratio. This indicated that Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio affected the catalytic activity of both neat HTs and Ag–Cu/HTs. The effect may be due to the change in acid/basic property of catalysts ascribing from the change in Mg
Al molar ratio affected the catalytic activity of both neat HTs and Ag–Cu/HTs. The effect may be due to the change in acid/basic property of catalysts ascribing from the change in Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio.11,25
Al molar ratio.11,25
The catalytic activity of neat HTs and Ag–Cu/HTs was also compared each other. As shown in Fig. 4, the yield to cinnamyl acetoacetate in the presence of Ag–Cu/HTs was far higher than that in the presence of neat HTs. For example, the yield was 50% for neat HTs at 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of Mg
1 of Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio, whereas the yield was 97% for Ag–Cu/HTs at the same Mg
Al molar ratio, whereas the yield was 97% for Ag–Cu/HTs at the same Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio. This indicated that Ag–Cu/HTs was more active than neat HTs. Considering the difference of neat HTs and Ag–Cu/HTs in composition, it could be deduced that Ag–Cu promoted the activity of Ag–Cu/HTs. For neat HTs, the active sites were obviously the acid/basic sites. However, for Ag–Cu/HTs, it was difficult to point out the main active sites since Ag–Cu metal sites and acid/basic sites were coexisted. In order to clarify the role of Ag–Cu sites, other three supports, i.e., AC, MgO, and γ-Al2O3, were investigated. As shown in Table 2, the supported Ag–Cu catalysts were more active than their corresponding neat supports, especially for AC supports. The yield to cinnamyl acetoacetate was 18% for neat AC, whereas the yield was 85% for Ag–Cu/AC. There were little acid/basic sites on neat AC, which explained its low activity. This indicated that Ag–Cu were the main active sites of Ag–Cu/AC. This also suggested that metal sites of Ag–Cu could catalyze transesterification in the absence of acid/basic sites.
Al molar ratio. This indicated that Ag–Cu/HTs was more active than neat HTs. Considering the difference of neat HTs and Ag–Cu/HTs in composition, it could be deduced that Ag–Cu promoted the activity of Ag–Cu/HTs. For neat HTs, the active sites were obviously the acid/basic sites. However, for Ag–Cu/HTs, it was difficult to point out the main active sites since Ag–Cu metal sites and acid/basic sites were coexisted. In order to clarify the role of Ag–Cu sites, other three supports, i.e., AC, MgO, and γ-Al2O3, were investigated. As shown in Table 2, the supported Ag–Cu catalysts were more active than their corresponding neat supports, especially for AC supports. The yield to cinnamyl acetoacetate was 18% for neat AC, whereas the yield was 85% for Ag–Cu/AC. There were little acid/basic sites on neat AC, which explained its low activity. This indicated that Ag–Cu were the main active sites of Ag–Cu/AC. This also suggested that metal sites of Ag–Cu could catalyze transesterification in the absence of acid/basic sites.
| Entry | Catalysts | Yield to cinnamyl acetoacetate (%) |
|---|---|---|
| a Reaction conditions: cinnamyl alcohol (11.0 mmol), ethyl acetoacetate (11.0 mmol), DMF (1.5 mL), catalyst (0.06 g), loading amount (4 wt%), 100 °C, reaction time of 24 h. | ||
| 1 | Blank | 15 |
| 2 | AC | 17 |
| 3 | Ag–Cu/AC | 85 |
| 4 | MgO | 32 |
| 5 | Ag–Cu/MgO | 42 |
| 6 | γ-Al2O3 | 44 |
| 7 | Ag–Cu/γ-Al2O3 | 53 |
3.3. The influence of Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) Cu molar ratio
Cu molar ratio
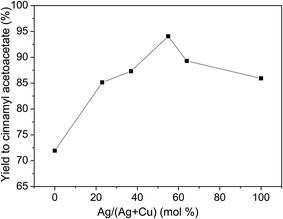
The effect of Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu molar ratio was investigated. As shown in Fig. 5, Ag
Cu molar ratio was investigated. As shown in Fig. 5, Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu molar ratio affected the yield to cinnamyl acetoacetate. When monometallic Cu/HTs was used as catalyst, the yield was 72%. With the increase of Ag
Cu molar ratio affected the yield to cinnamyl acetoacetate. When monometallic Cu/HTs was used as catalyst, the yield was 72%. With the increase of Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu molar ratio, the yield was increased. When Ag content was 55%, i.e., 1.2
Cu molar ratio, the yield was increased. When Ag content was 55%, i.e., 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of Ag
1 of Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu molar ratio, the yield reached the maximum of 94%. Above 55% of Ag content, the yield decreased. It went down to 86% when monometallic Ag/HTs was used as catalysts. This indicated that bimetallic Ag–Cu nanoparticles were more active than monometallic Ag or Cu nanoparticles. The high activity of bimetallic Ag–Cu nanoparticles may be ascribed to the interaction between Cu and Ag nanoparticles.
Cu molar ratio, the yield reached the maximum of 94%. Above 55% of Ag content, the yield decreased. It went down to 86% when monometallic Ag/HTs was used as catalysts. This indicated that bimetallic Ag–Cu nanoparticles were more active than monometallic Ag or Cu nanoparticles. The high activity of bimetallic Ag–Cu nanoparticles may be ascribed to the interaction between Cu and Ag nanoparticles.
The structure and property of Ag–Cu bimetallic nanoparticles have been reported in literature. Chen et al. synthesized Ag–Cu nanoparticles in the range of 12–30 nm with the assistance of microwave irradiation.42 Czaplinska et al. studied the effect of metal loading on bimetallic Ag–Cu/SBA-15 system.43 Chattopadhyay et al. have synthesized Ag–Cu alloy nanoparticles of four different compositions by using the laser ablation technique. Following this, they reported a morphological transition in the nanoparticles from a normal two-phase microstructure to a structure with random segregation and finally a core–shell structure at small sizes as a function of Cu concentration.44 Lee group investigated the enhanced resistance to oxidation by a combined experimental and theoretical studied.41 They calculated the atomic charge of the Ag–Cu bimetallic nanoparticles using Mulliken charge analysis, and found that there was electron transfer from Cu to Ag within these bimetallic nanoparticles. The electron transfer from Cu to Ag within these bimetallic nanoparticles allows far better resistance to oxidation than monometallic Cu nanoparticles. On the basis of the Gibbs free energy model, Peng et al. modeled the phase stability of janus, core–shell, and alloyed Ag–Cu nanoparticles.45 The results from the literature about Ag–Cu nanoparticles indicated that the structure of Ag–Cu nanoparticles were complicated, which ranged from Janus, core–shell, to alloy, and were affected by the size of nanoparticles, metal loading, and temperature. The results from the above literature also showed that the structure of Ag–Cu nanoparticles could not be fully characterized using conventional techniques. Owing to the complication in the structure of Ag–Cu nanoparticles, effect of the interaction between Cu and Ag nanoparticles on their catalytic performance will be further investigated in our future work.
3.4. Effect of reaction conditions
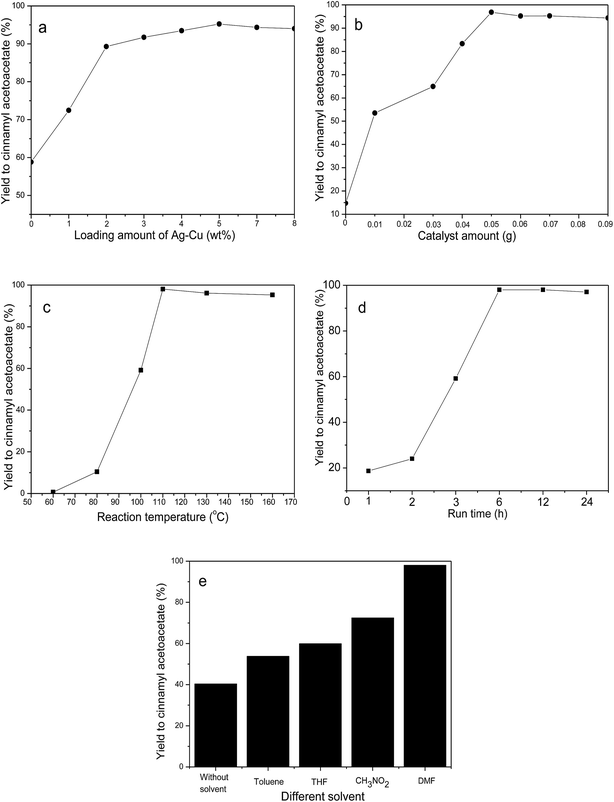
The effect of reaction conditions, including loading amount of Ag–Cu, catalyst amount, reaction temperature and time, and solvents, was investigated. As shown in Fig. 6, the optimum reaction conditions involved using DMF solvent and 0.05 g of 5 wt% Ag–Cu/HTs catalyst in a reaction at a temperature of 110 °C that lasted 6 h. Under these conditions the yield to cinnamyl acetoacetate approached 98% for the transesterification of cinnamyl alcohol and ethyl acetoacetate.3.5. The reusability of Ag–Cu/HTs
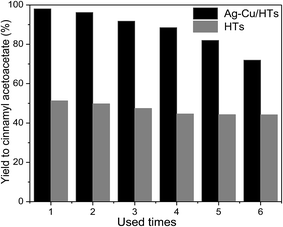
In order to verify whether the Ag–Cu/HTs catalysts were truly heterogeneous, a heterogeneity test was conducted. The catalyst was removed by filtration at half the specified reaction time period. Then, the filtrate was allowed to react under same conditions. The yield to cinnamyl acetoacetate was 17%, which was similar with that of the blank (Table 2, entry 1). This suggested that nature of Ag–Cu/HTs catalysts was truly heterogeneous.As shown in Fig. 7, the reusability of neat HTs and Ag–Cu/HTs were studied for transesterification of cinnamyl alcohol and ethyl acetoacetate. The catalysts were recovered after each run by centrifugation and washing with DMF for five times. Using Ag–Cu/HTs as catalyst, the yield to cinnamyl acetoacetate decreased slowly with number of cycles. The yield decrease from 99% for the fresh to 90% for the used for four times. In the presence of neat HTs, the yield did not apparently change with number of cycles. This indicates that the deactivation of Ag–Cu/HTs may be due to the change in the property of Ag–Cu nanoparticles. The leaching of Ag–Cu nanoparticles was investigated by AAS after each run. Negligible leaching was observed in the reaction solution. The observed leaching of Ag–Cu nanoparticles in reaction solution was 0.0015 wt% of its total amount. However, leaching was found in washing solvents. The leaching in the washing solvents may be due to the high rotation speed (3000 rpm) of centrifuge. After each run, the catalyst was washed by DMF solvents and separated by centrifuge for 5 times. The determined leaching of Ag–Cu nanoparticles for each washing was 0.43 wt% of its total amount. XRD analysis shows that there was no apparent change in the structure of Ag–Cu/HTs after used for six times. However, TEM analysis shows that a little part of used Ag–Cu nanoparticle agglomerated. Therefore, the deactivation of Ag–Cu/HTs may be due to agglomeration of part Ag–Cu nanoparticle and the loss of Ag–Cu nanoparticle in washing solvents.
3.6. Transesterification between various alcohols and different esters
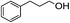
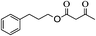
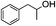
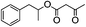
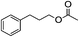
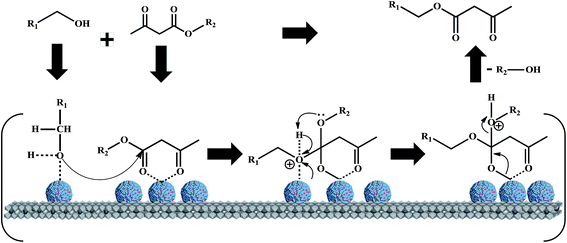
With the suitable reaction conditions established, the scope of substrates was then investigated (Tables 3 and 4). Very high yields (97%) of the corresponding products of transesterification were obtained from ethyl acetoacetate and aromatic benzyl alcohols (Table 3, entry 1). It was also found that the yield for allylic alcohol was far higher than that for the corresponding aliphatic alcohols. For example, the yield was 98% at 6 h of reaction time for cinnamyl alcohol (Table 3, entry 2), while the yield was 89% at 24 h for 3-phenylpropanol (Table 3, entry 3). For the other example, the yield for allyl alcohol was 88% (Table 3, entry 6), whereas the yield was 66% for n-propanol (Table 3, entry 7). The relation between yield in transesterification and the structure of alcohol was in accordance with that between yield in dehydrogenation and the structure of alcohol in the presence of Cu catalyst.46 This demonstrated the role of Cu sites in activating the O–H bond in the alcohol.Notably, the yield for β-ketoesters was far higher than that for the corresponding esters without the β-keto substituent (Table 4). For example, the yield was 97% at 5 h of reaction time for transesterification between ethyl acetoacetate (β-ketoester) and benzyl alcohols (Table 3, entry 1), whereas the yield only reached 80% at 24 h for the corresponding transesterification between ethyl acetate (ester without the β-keto substituent) and benzyl alcohols (Table 4, entry 1). Ag–Cu has vacant orbitals, and β-ketoesters have two carbonyl group. The Ag–Cu may react with β-ketoesters to form a cyclic intermediate through the interaction between the vacant orbits and two carbonyl oxygen atoms.9 This interaction may explain the high conversion of β-ketoesters. Based on the role of Ag–Cu nanoparticles and property of β-ketoesters, the reaction mechanism involved in the transesterification are proposed and shown in Scheme 2.
4. Conclusions
Ag–Cu metal site was proved to be active in catalyzing the transesterification between β-ketoester and various alcohols. The bimetallic Ag–Cu nanoparticles were more active that monometallic Ag or Cu nanoparticles. The high activity of bimetallic Ag–Cu nanoparticles may be ascribed to the interaction between Cu and Ag nanoparticles. The role of metal sites was ascribed to its activation to both the alcohol and β-ketoester. This work provided a successful try of applying metal catalysts to transesterification of β-ketoester and alcohols. Considering the diversity in metal catalysts, it is potential to develop more efficient catalysts through tuning the structure and types of metal catalysts. On one side, this work extended the application of Ag–Cu catalysts; on the other side, it introduced a new type of catalysts to transesterification.5. Characterization of the products
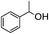
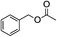
5.1. Benzyl 3-oxobutanoate (Table 2, entry 1)
1H-NMR (600 MHz, CDCl3): δ = 7.28–7.43 (m, 5H, Ph), δ = 5.18 (s, 2H, PhCH2), δ = 3.49 (s, 2H, COCH2CO), δ = 2.25 (s, 3H, COCH3). 13C-NMR (600 MHz, CDCl3): δ = 30.25, δ = 50.06, δ = 66.92, δ = 128.14, δ = 128.65, δ = 135.13, δ = 166.95, δ = 200.11.5.2. Cinnamyl acetate (Table 2, entry 2)
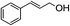
1H-NMR (600 MHz, CDCl3): δ = 7.22–7.42 (m, 5H, Ph), δ = 6.67 (d, J = 24.0 Hz, H, PhCH), δ = 6.27 (dt, J = 24.0 Hz, 9.6 Hz, H, CHCH2), δ = 4.80 (dd, J = 9.6 Hz, 1.8 Hz, 2H, CH2O), δ = 3.49 (s, 2H, COCH2CO), δ = 2.27 (s, 3H, COCH3). 13C-NMR (600 MHz, CDCl3): δ = 29.69, δ = 49.49, δ = 65.36, δ = 122.36, δ = 126.34, δ = 127.85, δ = 128.33, δ = 134.127, δ = 135.78, δ = 166.62, δ = 200.25.5.3. 3-Phenylpropyl 3-oxobutanoate (Table 2, entry 3)
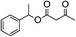
1H-NMR (600 MHz, CDCl3): δ = 7.13–7.29 (m, 5H, Ph), δ = 4.16 (m, 2H, CH2O), δ = 3.46 (s, 2H, COCH2CO), δ = 2.69 (s, 2H, PhCH2), δ = 2.28 (s, 3H, COCH3), δ = 1.98 (m, 2H, CH2CH2O). 13C-NMR (600 MHz, CDCl3): δ = 30.16, δ = 32.40, δ = 50.05, δ = 67.15, δ = 123.71, δ = 128.59, δ = 135.30, δ = 166.92, δ = 200.30.5.4. 1-Phenyl-2-propanolyl 3-oxobutanoate (Table 2, entry 4)
1H-NMR (600 MHz, CDCl3): δ = 7.17–7.30 (m, 5H, Ph), δ = 5.17 (s, H, CH), δ = 3.41 (s, 2H, COCH2CO), δ = 2.90 (m, 2H, PhCH2), δ = 2.28 (s, 3H, COCH3), δ = 1.25 (m, 3H, CHCH3). 13C-NMR (600 MHz, CDCl3): δ = 21.94, δ = 30.16, δ = 43.56, δ = 50.06, δ = 73.96, δ = 128.65, δ = 135.30, δ = 166.89, δ = 200.34.5.5. 1-Phenylethyl 3-oxobutanoate (Table 2, entry 5)
1H-NMR (600 MHz, CDCl3): δ = 7.19–7.41 (m, 5H, Ph), δ = 5.92 (dt, J = 10.80 Hz, 2.4 Hz, H, CH), δ = 3.44 (s, 2H, COCH2CO), δ = 2.26 (s, 3H, COCH3), δ = 1.47 (s, 3H, CHCH3). 13C-NMR (600 MHz, CDCl3): δ = 21.91, δ = 30.20, δ = 50.01, δ = 73.92, δ = 128.64, δ = 130.21, δ = 135.31, δ = 166.96, δ = 200.32.5.6. (E)-But-2-en-1-yl 3-oxobutanoate (Table 2, entry 6)
1H-NMR (600 MHz, CDCl3): δ = 5.89 (m, H, CH3CH), δ = 5.79 (m, H, CHCH2), δ = 4.57 (s, 2H, CH2O), δ = 3.46 (s, 2H, COCH2CO), δ = 2.27 (s, 3H, COCH3), δ = 1.72 (d, J = 5.4 Hz, 3H, CH3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH). 13C-NMR (600 MHz, CDCl3): δ = 17.16, δ = 30.12, δ = 49.97, δ = 65.94, δ = 118.90, δ = 131.53, δ = 166.75, δ = 200.33.
CH). 13C-NMR (600 MHz, CDCl3): δ = 17.16, δ = 30.12, δ = 49.97, δ = 65.94, δ = 118.90, δ = 131.53, δ = 166.75, δ = 200.33.
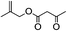
5.7. n-Butylacetoacetate (Table 2, entry 7)
1H-NMR (600 MHz, CDCl3): δ = 4.14 (m, 2H, CH2O), δ = 3.45 (s, 2H, COCH2CO), δ = 2.27 (s, 3H, COCH3), δ = 1.62 (m, 2H, CH2CH2O), δ = 1.39 (m, 2H, CH3CH2), δ = 0.94 (t, J = 7.2 Hz, 3H, CH3CH2). 13C-NMR (600 MHz, CDCl3): δ = 13.69, δ = 18.80, δ = 30.07, δ = 30.54, δ = 50.06, δ = 65.56, δ = 167.18, δ = 200.64.5.8. Acetoacetic acid allyl ester (Table 2, entry 8)
1H-NMR (600 MHz, CDCl3): δ = 5.93 (m, H, CCH3), δ = 5.36 (m, H, trans-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHH), δ = 5.26 (m, H, cis-CH
CHH), δ = 5.26 (m, H, cis-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHH), δ = 4.64 (s, 2H, CH2O), δ = 3.50 (s, 2H, COCH2CO), δ = 2.28 (s, 3H, COCH3). 13C-NMR (600 MHz, CDCl3): δ = 29.72, δ = 49.83, δ = 66.09, δ = 118.89, δ = 131.37, δ = 166.69, δ = 200.11.
CHH), δ = 4.64 (s, 2H, CH2O), δ = 3.50 (s, 2H, COCH2CO), δ = 2.28 (s, 3H, COCH3). 13C-NMR (600 MHz, CDCl3): δ = 29.72, δ = 49.83, δ = 66.09, δ = 118.89, δ = 131.37, δ = 166.69, δ = 200.11.
5.9. Propyl 3-oxobutanoate (Table 2, entry 9)
1H-NMR (600 MHz, CDCl3): δ = 4.10 (m, 2H, CH2O), δ = 3.46 (s, 2H, COCH2CO), δ = 2.28 (s, 3H, COCH3), δ = 1.68 (m, 2H, CH3CH2), δ = 0.95 (t, J = 7.8 Hz, 3H, CH3CH2). 13C-NMR (600 MHz, CDCl3): δ = 10.14, δ = 21.82, δ = 29.95, δ = 50.06, δ = 66.91, δ = 166.98, δ = 200.64.5.10. 2-Methylallyl 3-oxobutanoate (Table 2, entry 10)
1H-NMR (600 MHz, CDCl3): δ = 4.86 (m, 2H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), δ = 4.57 (s, 2H, CCH2), δ = 3.49 (s, 2H, COCH2CO), δ = 2.29 (s, 3H, COCH3), δ = 1.75 (m, 3H, CCH3). 13C-NMR (600 MHz, CDCl3): δ = 19.87, δ = 30.14, δ = 50.44, δ = 101.74, δ = 152.04, δ = 166.77, δ = 200.32.
C), δ = 4.57 (s, 2H, CCH2), δ = 3.49 (s, 2H, COCH2CO), δ = 2.29 (s, 3H, COCH3), δ = 1.75 (m, 3H, CCH3). 13C-NMR (600 MHz, CDCl3): δ = 19.87, δ = 30.14, δ = 50.44, δ = 101.74, δ = 152.04, δ = 166.77, δ = 200.32.
5.11. n-Hexyl acetylacetate (Table 2, entry 11)
1H-NMR (600 MHz, CDCl3): δ = 4.14 (t, J = 6.6 Hz, 2H, CH2O), δ = 3.45 (s, 2H, COCH2CO), δ = 2.28 (s, 3H, COCH3), δ = 1.96 (m, 2H, CH2CH2O), δ = 1.66 (m, 2H, CH2CH2CH2O), δ = 1.34 (m, 2H, CH2CH2CH2CH2O), δ = 1.30 (s, 2H, CH3CH2), δ = 0.89 (t, J = 6.6 Hz, 3H, CH3CH2). 13C-NMR (600 MHz, CDCl3): δ = 13.98, δ = 22.69, δ = 25.41, δ = 28.1, δ = 29.99, δ = 31.39, δ = 49.88, δ = 65.59, δ = 167.23, δ = 200.68.5.12. Acetic acid benzyl ester (Table 3, entry 1)
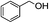
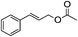
1H-NMR (600 MHz, CDCl3): δ = 7.26–7.39 (m, 5H, Ph), δ = 5.10 (s, 2H, PhCH2), δ = 2.09 (s, 3H, COCH3). 13C-NMR (600 MHz, CDCl3): δ = 20.99, δ = 66.31, δ = 128.28, δ = 128.59, δ = 136.02, δ = 170.84.5.13. Cinnamyl acetate (Table 3, entry 2)
1H-NMR (600 MHz, CDCl3): δ = 7.21–7.39 (m, 5H, Ph), δ = 6.65 (d, J = 12.0 Hz, H, PhCH), δ = 6.28 (dt, J = 24.0 Hz, 6 Hz, H, CHCH2), δ = 4.71 (dd, J = 6 Hz, 0.6 Hz, 2H, CH2O), δ = 2.09 (s, 3H, COCH3). 13C-NMR (600 MHz, CDCl3): δ = 20.99, δ = 65.33, δ = 123.23, δ = 126.78, δ = 128.10, δ = 128.63, δ = 134.38, δ = 136.25, δ = 170.76.5.14. Phenylpropyl acetate (Table 3, entry 3)
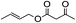
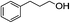
1H-NMR (600 MHz, CDCl3): δ = 7.15–7.35 (m, 5H, Ph), δ = 4.08 (s, 2H, CH2O), δ = 2.68 (m, 2H, PhCH2), δ = 2.04 (s, 3H, COCH3), δ = 1.95 (m, 2H, CH2CH2O). 13C-NMR (600 MHz, CDCl3): δ = 20.98, δ = 29.95, δ = 32.13, δ = 63.68, δ = 125.71, δ = 128.66, δ = 141.16, δ = 171.04.5.15. Acetic acid 2-butenyl ester (Table 3, entry 4)
1H-NMR (600 MHz, CDCl3): δ = 5.79 (m, H, CH3CH), δ = 5.59 (m, H, CHCH2), δ = 4.50 (d, J = 6.60 Hz, 2H, CH2O), δ = 2.05 (s, 3H, COCH3), δ = 1.73 (m, 3H, CH3CH). 13C-NMR (600 MHz, CDCl3): δ = 17.71, δ = 20.95, δ = 65.34, δ = 125.09, δ = 131.38, δ = 170.75.5.16. n-Propyl acetate (Table 3, entry 5)
1H-NMR (600 MHz, CDCl3): δ = 4.20 (t, J = 7.2 Hz, 2H, CH2O), δ = 2.05 (s, 3H, COCH3), δ = 1.65 (m, 2H, CH3CH2), δ = 0.94 (t, J = 7.8 Hz, 3H, CH3CH2). 13C-NMR (600 MHz, CDCl3): δ = 10.37, δ = 20.47, δ = 21.81, δ = 65.84, δ = 171.09.5.17. n-Propyl acetate (Table 3, entry 6)
1H-NMR (600 MHz, CDCl3): δ = 4.06 (t, J = 6.6 Hz, 2H, CH2O), δ = 2.05 (s, 3H, COCH3), δ = 1.61 (m, 2H, CH2CH2O), δ = 1.39 (m, 2H, CH3CH2), δ = 0.94 (t, J = 7.2 Hz, 3H, CH3CH2). 13C-NMR (600 MHz, CDCl3): δ = 13.64, δ = 19.09, δ = 20.76, δ = 30.55, δ = 63.98, δ = 171.05.Acknowledgements
This work was supported by projects funded by the Major Research Plan of National Natural Science Foundation of China (Program No. 91545130), the Natural Science Basic Research Plan in Shaanxi Province of China (Program No. 2013JM2003), and by the Fundamental Research Funds for the Central Universities (Program No. GK201505002).References
- S. Benetti and R. Romagnoli, Chem. Rev., 1995, 95, 1065 CrossRef CAS.
- F. Xie, F. Yan, M. Chen and M. Zhang, RSC Adv., 2014, 4, 29502 RSC.
- P. Baburajan and K. P. Elango, Tetrahedron Lett., 2014, 55, 3525 CrossRef CAS.
- R. C. Brinkerhoff, H. F. Tarazona, P. M. Oliveira, D. C. Flores, C. D. R. M. D'Oca, D. Russowsky and M. G. M. D'Oca, RSC Adv., 2014, 4, 49556 RSC.
- B. P. Bandgar, L. S. Uppalla and V. S. Sadavarte, Green Chem., 2001, 3, 39 RSC.
- J. S. Yadav, B. V. S. Reddy, A. D. Krishna, C. S. Reddy and A. V. Narsaiah, J. Mol. Catal. A: Chem., 2007, 261, 93 CrossRef CAS.
- M. I. de Sairre, E. S. Bronze-Uhle and P. M. Donate, Tetrahedron Lett., 2005, 46, 2705 CrossRef CAS.
- S. Benetti, R. Romagnoli, C. De Risi, G. Spalluto and V. Zanirato, Chem. Rev., 1995, 95, 1065 CrossRef CAS.
- G. Krishnaiah, B. Sandeep, D. Kondhare, K. C. Rajanna, J. N. Reddy, Y. R. Rao and P. K. Zhubaidha, Tetrahedron Lett., 2013, 54, 703 CrossRef CAS.
- M. Liao and J. Wang, Tetrahedron Lett., 2006, 47, 8859 CrossRef CAS.
- J. Otera, Chem. Rev., 1993, 93, 1449 CrossRef CAS.
- G. Krishnaiah, K. C. Rajanna, K. Rajendar Reddy, M. Satish Kumar, P. Srinivas and Y. Rajeshwer Rao, Res. Chem. Intermed., 2015, 41, 2739 CrossRef CAS.
- B. P. Bandgar, V. S. Sadavarte and L. S. Uppalla, Synlett, 2001, 8, 1338 Search PubMed.
- B. C. Ranu, P. Dutta and A. Sarkar, J. Org. Chem., 1998, 63, 6027 CrossRef CAS PubMed.
- B. Wang, L. M. Yang and J. S. Suo, Tetrahedron Lett., 2003, 44, 5037 CrossRef CAS.
- P. Kumar and R. K. Pandey, Synlett, 2000, 31, 251 CrossRef.
- B. P. Bandgar, V. S. Sadavarte and L. S. Uppalla, Chem. Lett., 2001, 30, 894 CrossRef.
- T. Jin, S. Zhang and T. Li, Green Chem., 2002, 4, 32 RSC.
- R. K. Sharma and D. Rawat, J. Inorg. Organomet. Polym., 2011, 21, 619 CrossRef CAS.
- B. M. Reddy, V. R. Reddy and B. Manohar, Synth. Commun., 1999, 29, 1235 CrossRef CAS.
- S. P. Chavan, P. K. Zubaidha, S. W. Dantale, A. Keshavaraja, A. V. Ramaswamy and T. Ravindranathan, Tetrahedron Lett., 1996, 37, 233 CrossRef CAS.
- B. R. Madje, P. T. Patil, S. S. Shindalkar, S. B. Benjamin and M. S. Shingare, Catal. Commun., 2004, 5, 353–357 CrossRef CAS.
- D. E. Ponde, V. H. Deshpande, V. J. Bulbule, A. Sudalai and A. S. Gajare, J. Org. Chem., 1998, 63, 1058 CrossRef.
- A. Pericas, A. Shafir and A. Vallribera, Tetrahedron, 2008, 64, 9258 CrossRef CAS.
- B. M. Choudary, M. L. Kantam, C. V. Reddy, S. Aranganathan, P. L. Santhi and F. Figueras, J. Mol. Catal. A: Chem., 2000, 159, 411 CrossRef CAS.
- M. L. Kantam and P. Sreekanth, Catal. Lett., 2001, 77, 241 CrossRef CAS.
- M. Katiyar and A. Ali, J. Am. Oil Chem. Soc., 2015, 92, 623 CrossRef CAS.
- F. Guo, N. Wei, Z. Xiu and Z. Fang, Fuel, 2012, 93, 468 CrossRef CAS.
- C. Baldizzone, S. Mezzavilla, H. W. P. Carvalho, J. C. Meier, A. K. Schuppert, M. Heggen, C. Galeano, J. Grunwaldt, F. Schüth and K. J. J. Mayrhofer, Angew. Chem., Int. Ed., 2014, 53, 14250 CrossRef CAS PubMed.
- S. Guo, X. Zhang, W. Zhu, K. He, D. Su, A. Mendoza-Garcia, S. F. Ho, G. Lu and S. Sun, J. Am. Chem. Soc., 2014, 136, 15026 CrossRef CAS PubMed.
- M. Haruta, Angew. Chem., Int. Ed., 2014, 53, 52 CrossRef CAS PubMed.
- S. E. Davis, M. S. Ide and R. J. Davis, Green Chem., 2013, 15, 17–45 RSC.
- R. Das and D. Chakraborty, Appl. Organomet. Chem., 2012, 26, 140 CrossRef CAS.
- M. Gohain, V. Kumar, J. H. Tonder, H. C. Swart, O. M. Ntwaeaborwa and B. C. B. Bezuidenhoudt, RSC Adv., 2015, 5, 18972 RSC.
- C. L. Bracey, P. R. Ellis and G. J. Hutchings, Chem. Soc. Rev., 2009, 38, 2231 RSC.
- C. Xu, J. Sun, B. Zhao and Q. Liu, Appl. Catal., B, 2010, 99, 111 CrossRef CAS.
- G. L. Brett, Q. He, C. Hammond, P. J. Miedziak, N. Dimitratos, M. Sankar, A. A. Herzing, M. Conte, J. A. Lopez-Sanchez, C. J. Kiely, D. W. Knight, S. H. Taylor and G. J. Hutchings, Angew. Chem., Int. Ed., 2011, 50, 10136 CrossRef CAS PubMed.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- N. Schöwe, K. Bretz, T. Hennig, S. Schlüter and G. Deerberg, Ind. Eng. Chem. Res., 2015, 54, 1123 CrossRef.
- C. Xu, Y. Du and J. Yang, Appl. Catal., B, 2015, 164, 334 CrossRef CAS.
- N. R. Kim, K. Shin, I. Jung, M. Shim and H. M. Lee, J. Phys. Chem. C, 2014, 118, 26324 CAS.
- Z. Chen, D. Mochizuki, M. M. Maitani and Y. Wada, Nanotechnology, 2013, 24, 265602 CrossRef PubMed.
- J. Czaplinska, I. Sobczak and M. Ziolek, J. Phys. Chem. C, 2014, 118, 12796 CAS.
- K. D. Malviya and K. Chattopadhyay, J. Phys. Chem. C, 2014, 118, 13228 CAS.
- H. Peng, W. Qi, S. Li and W. Ji, J. Phys. Chem. C, 2015, 119, 2186 CAS.
- D. Damodara, R. Arundhathi and P. R. Likhar, Adv. Synth. Catal., 2014, 356, 189 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra00467a |
| This journal is © The Royal Society of Chemistry 2016 |