Band gap engineering of MnO2 through in situ Al-doping for applicable pseudocapacitors†
Tianqi Li‡
a,
Jiabin Wu‡b,
Xu Xiaoa,
Bingyan Zhanga,
Zhimi Hua,
Jun Zhoua,
Peihua Yanga,
Xun Chena,
Bo Wanga and
Liang Huang*a
aWuhan National Laboratory for Optoelectronics, School of Optical and Electronic Information, Huazhong University of Science and Technology, Wuhan 430074, P. R. Chinacn. E-mail: huangliang421@hust.edu.cn
bSchool of Materials Science and Engineering, Wuhan University of Technology, Wuhan 430074, P. R. China
First published on 27th January 2016
Abstract
Band gap engineering was achieved by in situ doping method for high electrical conductivity and chemical activity of MnO2. By in situ releasing and adsorption during electrodeposition, Al3+ with close ion radius to Mn4+ could replace the position of Mn4+ in MnO2. The in situ doping process brings impurity level in MnO2 and changes the energy band structure. The narrower band gap of MnO2 after Al doping with higher electron concentration on conduction band could improve the conductivity of MnO2. The specific capacitance of Al-doped MnO2 achieves 430.6 F g−1 which is almost 2.5 times of the capacitance of initial MnO2 (177 F g−1), shedding light on its practical applications.
Introduction
Pseudocapacitors (charging time ranging from 10 s to 10 min) have drawn increasing attention in addressing the emerging energy demands.1,2 By virtue of the reversible and fast redox reaction, high theoretical capacitance (1370 F g−1), low cost, environmental benignancy and natural abundance, manganese dioxide (MnO2) has been one of the most promising electrode materials.3–6 However, MnO2 loses 70% capacitance with increasing load amount because of its low electronic conductivity (10−6 to 10−3 S cm−1).7 In order to enhance the conductivity of MnO2, many works have been focusing on engineering MnO2 to nanostructure for shortening the electron transport path, or hybridized with conductive agents such as ZnO,8 carbon particles,9 CNT10,11 and graphene12,13 to form MnO2/carbon composite. Nevertheless, extra carbon does not result in the best energy utilization in terms of relatively low capacitance of carbon due to its charge storage mechanism of electrical double layer adsorption and low surface area. Generally, electrochemical performance depends on ion diffusion, electron transport and charge transfer. Since the high electric conductivity of aqueous electrolyte could take place fast reaction near the electrode surface, the ion diffusion control is negligible. Accordingly, electrochemical performance is just controlled by electron transport which is limited by carrier concentration/carrier mobility and charge transfer. For semiconductor, the band gap plays a decisive role in transition energy. Lower transition energy brings higher concentration of free movable electron in conduction band. As a result, the improvement of both electron transport and electrochemical activity could be realized by tuning band gap.Recently work demonstrated doping is a effective method to adjust the band gap and tune the conductivity of semiconductors14,15 Chen and his co-workers proved that the Au-doping was effective for improving conductivity.16 Distinctly, Au is a free electron noble metal can't occur equilibrium electron-doping. While active metals forming cation-doping could change the morphology and active area of matrix. By changing electron distribution, cation-doping could also enhance the conductivity17 such as Ag-doping,18 Mg-doping,19 Cu-doping19 and Fe-doping,20 but the mechanism of doping process is obscure. Generally, the cations can stably exist in the crystal in two ways: replacement or insertion. The insertion doping similar as cation intercalation may change crystal structure.21 It is well-known that the replacing-doping will change the bonding energy via producing the new molecular orbits which introduces impurity level in semiconductors to narrow down band gap.22 Specially, Al3+ may be the best cation for replace-doping because its ion radius (53.5 pm) is close to the ion radius of Mn4+ (53 pm). The Al3+ cation has different valence state with Mn4+ may cause an oxygen vacancy and hence improve electric conductivity of the MnO2. This phenomenon was proved in our previous work by using hydrothermal method to synthesize the Al-doped MnO2. The conductivity could be effectively tailored by different Al doping content. However, it is tough to clearly understand the Al doping mechanism because the difficult controllable growth process in the hydrothermal method. For deeply understanding of doping process, electrodeposition is employed to discuss the influence of Al ion on the energy band structure of MnO2 by tracking the concentration of ions through ultramicroelectrode.
Herein, we use electrodeposition method to synthesize Al-doped MnO2 nanoflakes. According to the synthesis, the electrodeposition of MnO2 occurs in positive potential.23 In order to realize the Al-doping process, [AlF6]3− is chosen to be the electronegative Al source. The as-obtained Al-doped MnO2 (ADM) shows narrower band gap and much better electrochemical performance than as-synthesized MnO2 (ASM). The band gap of MnO2 changes from 2.57 eV to 2.41 eV after Al-doping. The specific capacitance of ADM achieves 430.6 F g−1 at 2 mV s−1 and 225 F g−1 at 200 mV s−1, while the capacitance of the ASM is 177.7 F g−1 at 2 mV s−1 and 86.5 F g−1 at 200 mV s−1 under the same condition.
Experimental
All the reagents used in the experiment were of analytical grade and used without further purification.Synthesis of Al-doped MnO2
The electrodeposition was treated as a facile and large-scale way to synthesize MnO2. Typically, 0.01 M MnAc2 and 0.02 M NH4Ac aqueous solution were mixed with 10 vol% dimethyl sulfoxide solution under stirring. Then, 0.001 M Na3AlF6 was slowly added into the mixed solution. The current density used for electrodeposition was 0.6 mA cm−2 on platinum sheet and the counter electrode was graphite. After electrodeposition for 10 min, the MnO2 was washed and scraped from platinum sheet and dried at 80 °C for 12 h.Preparation of the electrodes
The electrodes were prepared by mixing 70 wt% Al-doped MnO2 powder, 25 wt% acetylene black (Alfa Aesar, 99.9%), and 5 wt% poly (tetrafluoroethylene) (PTFE) in ethanol. The resulting solution was stirred overnight, and volatilized the ethanol at 80 °C. The dried mixture was roll-pressed into a 20 μm-thick film on a flat glass surface, which has typically 1 cm2 surface area and about 0.5 mg mass. The electrodes were dried overnight at 60 °C before testing.Physical and electrochemical characterization
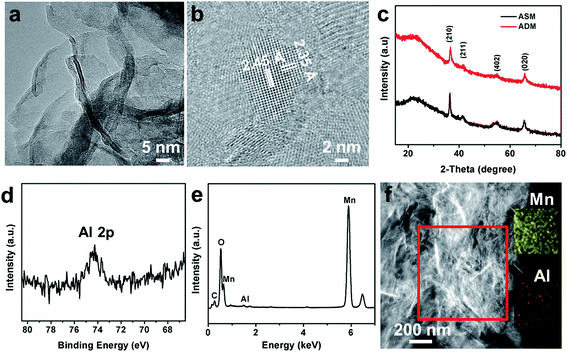
The microstructural properties of electrode materials were characterized by X-ray diffraction using the Cu Kα radiation (λ = 1.5418 Å) (XRD, Philips X’ Pert Pro), field-emission SEM (FE-SEM, FEI Nova 450 Nano), TEM (HRTEM, TECNAI, Titan) equipped with an energy-dispersive X-ray spectroscopy (EDS) detector, X-ray photoelectron spectroscope (XPS, AXIS-ULTRA DLD-600W), UV-visible absorption spectroscopy (UV-vis, Shimadzu, UV-2550). The scanning electrochemical microscope (SECM) was characterized by CHI 920C electrochemical workstation (Chenhua, Shanghai). The electrochemical properties of the products were investigated with cyclic voltammetry (CV) and chronopotentiometry measurements employing a CHI 660D electrochemical workstation (Chenhua, Shanghai) and Autolab PGSTAT302N, and the electrochemical impedance spectroscopy (EIS) was measured by an Autolab PGSTAT302N at a frequency ranging from 100 mHz to 10 kHz with a potential amplitude of 5 mV at open-circuit potential. The mass of electrode materials was measured by a microbalance (CPA225D, Sartorius) with an accuracy of 0.01 mg. The element content of Al was measured by inductively coupled plasma emission spectrum (ICP-OES, Optima 4300DV). N2 adsorption–desorption isotherms were performed on a Micrometrics ASAP 2000. The capacitances were calculated by integrating the discharge portions of the cyclic voltammograms (CVs).Results and discussion
The electrochemical reaction occurred on the platinum sheet where is the place that Mn2+ lost its electron and deposited on the substrate are following this equation:| Mn2+ − 2e− + 2H2O = MnO2 + 4H+ |
The H+ from the reaction may lower the value of pH near the surface of substrate. With moving the probe from the electrolyte to electrode surface, the value of pH rose up as tested by SECM based on the hydrogen evolution current (Fig. 1a). The Al source of Na3AlF6 could ionize to Na+ and [AlF6]3− in the aqueous solution. [AlF6]3− is unstable in acidic solution which may releases the Al3+ cation. Al3+ cation could occupy the depositional place by adsorption on where the MnO2 deposited, leading to high H+ content and low value of pH. According to following equations:
| [AlF6]3− + H+ = [AlF5]2− + HF |
| [AlF5]2− + H+ = [AlF4]1− + HF |
| [AlF4]1− + H+ = [AlF3] + HF |
| [AlF3] + H+ = [AlF2]1+ + HF |
| [AlF2]1+ + H+ = [AlF]2+ + HF |
| [AlF]2+ + H+ = Al3+ + HF |
| [AlF6]3− + 6H+ = Al3+ + 6HF Ka = 0.1749 |
To verify the content and distribution of Al in ADM, the TEM-mapping, EDS, XPS and ICP were used. The existence of Al was proved in Fig. 2d and e. As shown in the Fig. 2f, the elements of Mn and Al are distributed uniformly in ADM. The content of Al doping could be confirmed in ICP, which determined the ratio of Al over Mn was 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. The ratio of Al
100. The ratio of Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn in synthesized material was identical to the ratio of Al3+
Mn in synthesized material was identical to the ratio of Al3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn2+ on the surface during the electrodeposition. Accordingly, the reaction rates of immobilization of Al and deposition of Mn were the same, which showed in situ behavior of Al doping during electrodeposition.
Mn2+ on the surface during the electrodeposition. Accordingly, the reaction rates of immobilization of Al and deposition of Mn were the same, which showed in situ behavior of Al doping during electrodeposition.
Generally, doping would change the energy band structure.22 The band gap test was based on UV-vis absorption spectra. The optical band gap Eg for the semiconductor has been graphically determined from the equation (αEp)2 = K(Ep − Eg) (where α is the absorption coefficient, K is a constant, Ep is the discrete photo energy, and Eg is the band gap energy).24 The plots of absorbance versus Ep based on the direct transition for ADM and ASM were displayed in Fig. 3a, from which band gap was changed from 2.57 eV to 2.41 eV. The band gap of semiconductor corresponds to the energy difference between valance band and conduction band. From XPS spectra (Fig. 3b), the top of valance band is highest binding energy of electron fill and defines as the intercept near 0 eV on background. Valance band of MnO2 is Mn3d t2g state25 which keep consistent after Al-doping. It is inferred that Al-doping can introduce the impurity level in the MnO2 and conjectured as a new antibonding orbit. The molecular orbits are divided into bonding orbits and antibonding orbits, and the new antibonding with lower energy offered empty orbits between valance and conduction band. The new antibonding with no electron filling will not change the valance band and lead to shortened band gap. As widely known, XPS is used to study the surface of materials. Here, the results of ICP and XPS are identical, which benefits from the uniform distribution of Al in ADM and thin nanoflakes morphology (Fig. 2b). Hence the XPS analysis for surface could represent the whole material. After Al doping, the binding energy of O 1s was lower (Fig. 3d and e), leading to lower energy of Mn–O bond and brings a new antibonding orbits into band gap. Due to the low content of Al, the Al–O bond is indistinguishable. Thus, the O 1s peak is divided into two peaks which are denoted as structure oxygen and active oxygen. The structure oxygen with higher binding energy has lower concentration of outer electron. The active oxygen has lower binding energy, suggesting the oxygen atom has higher concentration of outer electron. After Al-doping, the active oxygen was increased. Accordingly, the high electron concentration is in favor of trapping/losing proton or Na+, which causes feasible capacitance of MnO2 by following equation:
| MnO2 + xA+ + xe− = MnOOA (A = H, Na, x = 0–1). |
With narrower band gap and more active oxygen, ADM should hold great potential for high electrochemical performance pseudocapacitors.
To verify this hypothesis, electrochemical performance of ASM and ADM was studied systematically using three-electrode system in 0.5 M Na2SO4 aqueous solution. Electrochemical impedance spectroscopy (EIS) was first employed to reveal the enhanced conductivity by Al doping. As shown in Fig. 4a, the diameter of the semicircle at the high frequency region of samples decreased from 24.1 Ω (ASM) to 8.4 Ω (ADM). Since the electrodes were prepared at the same conditions (areal mass loading, diameter, thickness, assembly, and test), the difference of contact resistance in these two cases can be ignored. Therefore, the difference in the semicircle resistance could be directly related to the conductivity differences of the electrode materials. After doping, the conductivity was great enhanced.
The cyclic voltammetry (CV) curves which performed under sweep rate from 2 mV s−1 to 200 mV s−1 were shown in Fig. 4b and S4.† According to the surface or near-surface reaction of MnO2, neither of them appeared redox peaks. Thus, the rectangular shape is deeply affected by equivalent series resistance (ESR) which is based on the conductivity of the electrode materials. Comparing the CV curves at 20 mV s−1 in Fig. 4b, ADM showed much better conductivity to keep the rectangular shape. Another notable feature is that the CV curves of ADM retained a symmetrical rectangular shape even at a scan rate of 200 mV s−1 (Fig. 4c), demonstrating rapid capacitive response which benefits from high conductivity. By integrating the discharge portions of the CVs, ADM showed much higher capacitance (430.6 F g−1) at the scan rate of 2 mV s−1, which was twice larger than ASM (177.7 F g−1). Moreover, the capacitance retention achieves almost 100% even after 5000 cycles at current density of 1 A g−1, suggesting the high stability of ADM (Fig. S4†). Based on above discussion, it could be concluded that band gap engineering is a rational and powerful method to in situ promote the electrochemical performance of MnO2 by enhancing both of conductivity and content of active oxygen.
Conclusion
In summary, the electric conductivity and electrochemical activity of MnO2 was obviously improved by band gap engineering. The band gap was narrowed down by in situ Al doping which distributed stably and uniformly in MnO2. The Al-doping brings shortened band gap and unchanged valance band by introducing antibonding orbitals. Accordingly, the concentration of free movable electron is increased, which enhances both conductivity and activity. Due to the high electrochemical activity, the capacitance of ADM achieved 430.6 F g−1, over twice of ASM (177.7 F g−1), while the high conductivity supports capacitance remain 52% with scan rate increasing from 2 mV s−1 to 200 mV s−1. The methodology developed in this study is not limited only to Al-doped MnO2 but can be applied to other cations doping to improve the conductivity of other metal oxide by tuning band gap. This strategy could be widely applied to energy storage, photocatalytic water splitting or electrocatalysis.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (61501215), the China Postdoctoral Science Foundation (2014M550390, 2015T80793) and Director Fund of WNLO. The authors thank G. Li, Dr L. Y. Li and Dr J. Su for the help in experiments. The authors also acknowledge support of the Analysis and Testing Center of Huazhong University of Science and Technology.References
- V. Augustyn, P. Simon and B. Dunn, Energy Environ. Sci., 2014, 7, 1597–1614 CAS.
- J. R. Miller and P. Simon, Science, 2008, 321, 651–652 CrossRef CAS PubMed.
- W. Wei, X. Cui, W. Chen and D. G. Ivey, Chem. Soc. Rev., 2011, 40, 1697–1721 RSC.
- Z. Yu, B. Duong, D. Abbitt and J. Thomas, Adv. Mater., 2013, 25, 3302–3306 CrossRef CAS PubMed.
- X. Lu, T. Zhai, X. Zhang, Y. Shen, L. Yuan, B. Hu, L. Gong, J. Chen, Y. Gao, J. Zhou, Y. Tong and Z. L. Wang, Adv. Mater., 2012, 24, 938–944 CrossRef CAS PubMed.
- P. Yang, Y. Ding, Z. Lin, Z. Chen, Y. Li, P. Qiang, M. Ebrahimi, W. Mai, C. P. Wong and Z. L. Wang, Nano Lett., 2014, 14, 731–736 CrossRef CAS PubMed.
- C. Xu, F. Kang, B. Li and H. Du, J. Mater. Res., 2010, 25, 1421–1432 CrossRef CAS.
- P. Yang, X. Xiao, Y. Li, Y. Ding, P. Qiang, X. Tan, W. Mai, Z. Lin, W. Wu, T. Li, H. Jin, P. Liu, J. Zhou, C. P. Wong and Z. L. Wang, ACS Nano, 2013, 7, 2617–2626 CrossRef CAS PubMed.
- L. Yuan, X. H. Lu, X. Xiao, T. Zhai, J. Dai, F. Zhang, B. Hu, X. Wang, L. Gong, J. Chen, C. Hu, Y. Tong, J. Zhou and Z. L. Wang, ACS Nano, 2012, 6, 656–661 CrossRef CAS PubMed.
- X. Li and B. Q. Wei, Nano Energy, 2012, 1, 479–487 CrossRef CAS.
- K.-W. Nam, C.-W. Lee, X.-Q. Yang, B. W. Cho, W.-S. Yoon and K.-B. Kim, J. Power Sources, 2009, 188, 323–331 CrossRef CAS.
- Y. He, W. Chen, X. Li, Z. Zhang, J. Fu, C. Zhao and E. Xie, ACS Nano, 2013, 7, 174–182 CrossRef CAS PubMed.
- G. Yu, L. Hu, N. Liu, H. Wang, M. Vosgueritchian, Y. Yang, Y. Cui and Z. Bao, Nano Lett., 2011, 11, 4438–4442 CrossRef CAS PubMed.
- Y. Li and H. Xie, Ionics, 2010, 16, 21–25 CrossRef CAS.
- G. Wang, G. Shao, L. Wang, J. Song, Z. Ma and T. Liu, Ionics, 2014, 20, 1367–1375 CrossRef CAS.
- J. Kang, A. Hirata, L. Kang, X. Zhang, Y. Hou, L. Chen, C. Li, T. Fujita, K. Akagi and M. Chen, Angew. Chem., Int. Ed. Engl., 2013, 52, 1664–1667 CrossRef CAS PubMed.
- W.-H. Ryu, D.-W. Han, W.-K. Kim and H.-S. Kwon, J. Nanopart. Res., 2011, 13, 4777–4784 CrossRef CAS.
- Y. H. Wang and I. Zhitomirsky, Mater. Lett., 2011, 65, 1759–1761 CrossRef CAS.
- A. M. Hashem, H. M. Abuzeid, N. Narayanan, H. Ehrenberg and C. M. Julien, Mater. Chem. Phys., 2011, 130, 33–38 CrossRef CAS.
- Y. Ma, C. Fang, B. Ding, G. Ji and J. Y. Lee, Adv. Mater., 2013, 25, 4646–4652 CrossRef CAS PubMed.
- O. Ghodbane, F. Ataherian, N.-L. Wu and F. Favier, J. Power Sources, 2012, 206, 454–462 CrossRef CAS.
- Z. Hu, X. Xiao, C. Chen, T. Li, L. Huang, C. Zhang, J. Su, L. Miao, J. Jiang, Y. Zhang and J. Zhou, Nano Energy, 2015, 11, 226–234 CrossRef CAS.
- X. Lu, D. Zheng, T. Zhai, Z. Liu, Y. Huang, S. Xie and Y. Tong, Energy Environ. Sci., 2011, 4, 2915–2921 CAS.
- S. Jana, S. Pande, A. K. Sinha, S. Sarkar, M. Pradhan, M. Basu, S. Saha and T. Pal, J. Phys. Chem. C, 2009, 113, 1386–1392 CAS.
- N. Sakai, Y. Ebina, K. Takada and T. Sasaki, J. Phys. Chem. B, 2005, 109, 9651–9655 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26830c |
| ‡ Authors with equal contribution. |
| This journal is © The Royal Society of Chemistry 2016 |