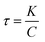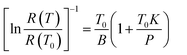DOI:
10.1039/C5RA26760A
(Paper)
RSC Adv., 2016,
6, 35118-35123
High performance of Ni0.9Mn1.8Mg0.3O4 spinel nanoceramic microbeads via inkjet printing and two step sintering†
Received
15th December 2015
, Accepted 1st April 2016
First published on 4th April 2016
Abstract
We report a simple and effective in situ inkjet printing method for the fabrication of nanostructured and highly dense Ni0.9Mn1.8Mg0.3O4 (NMM) spinel oxide nanoceramic microbeads materials. In our method, the ceramic inks are fabricated that single cubic spinel NMM nanocrystallines synthesized via co-precipitation method, disperse well in organic solution, and have shown perfect dispersibility and stability. After inkjet printing using these ceramic inks, NMM microbeads are sintered via two-step sintering (TSS) process at different temperatures. The sintering behavior, microstructure and electrical properties of NMM nanoceramic microbeads prepared via TSS process are investigated and characterized systematically. These results indicate that high performance of NMM microbeads with moderate resistance values (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 331–174
331–174![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 601 Ω), lower thermal time constant (58–147 ms), and higher material constant (4176–4309 K) are achieved by tuning the sintering temperatures.
601 Ω), lower thermal time constant (58–147 ms), and higher material constant (4176–4309 K) are achieved by tuning the sintering temperatures.
Introduction
The inkjet printing technique, recently, has played an increasingly important role in plenty of novel fields due to their exceptional technical advantages, such as fabrication flexibility, minimum tools, cost savings, environmental advantages, diverse designs, precise control of droplets quantity, and most important, good compatibility with any flexible substrates.1–3 Therefore, this technique utilizes in a variety of applications including microdosing drugs,4 functional films,5 photovoltaic devices,6 polymers,7 sensors8 and functional ceramics.9,10 There are numerous types of functional ceramic materials formulated into printable ceramic inks, including Al2O3,11 ZnO,12 TiO2,13 and BaTiO3.14 Furthermore, the inkjet print technique is particularly suitable for the preparation of micro functional ceramic devices. Ni0.9Mn1.8Mg0.3O4 (NMM) spinel oxides, as a typical functional ceramic materials,15,16 have attracted substantial attention in numerous industrial and domestic appliance fields due to its higher material constant B, lower time constants τ, as well as its better sensitivity to temperature change. Because of these benefits, NMM microbead ceramic materials have been widely utilized in some harsh environments such as aerospace and deep-sea fields. Therefore, the more straightforward method, three-dimensional inkjet printing is a mold-free manufacturing technique that NMM ceramic inks are directly and accurately inkjet-printed onto parallel Pt–Ir wires substrates and subsequently shrunk into NMM microbeads by the organic solution volatilization.
Among these numerous procedures, ceramic inks, as a critical process, have an essential effect on the inkjet printing fabrication of NMM ceramic materials.7–10 The ceramic inks need to control well these properties such as viscosity, surface tension and particle size to jet steadily. In addition, in order to avoid aggregation, ceramic inks must have excellent dispersibility, stability and the particle size has better lower than 100 nm. To overcome this problem, co-precipitation method has been demonstrated to be a promising and effective process for preparing NMM nanoceramic materials due to its numerous advantages17–19 mainly including low-aggregated, simple synthesis process, and controlling the particle size distribution and morphology.
In this paper, we report the fabrication process of highly dense NMM spinel microbeads using suitable ceramic inks as well as sintering process. TSS process, due to its simplicity and possibility to achieve the complete densification process at a relatively low temperature without pressure, has been demonstrated for a range of various ceramic systems such as Y2O3, MgAl2O4, and SiC.20–22 Unfortunately, to the best of our knowledge, there are still no reports of realizing high dense NMM microbeads fabricated via TSS method. Hence, we also discuss the effect of TSS process on the microstructure and electrical properties of NMM samples. High quality NMM microbeads are achieved for the first time with excellent performance of material constant B, time constants and moderate resistivity, through selecting the proper TSS parameters.
Experimental
Synthesis of NMM nanoparticles
In our previous study,15 the coprecipitation method was applied to synthesize the NMM ceramic inks required nanoparticles. Manganese(II) acetate tetrahydrate (C4H6MnO4·4H2O), nickel(II) acetate tetrahydrate (C4H6NiO4·4H2O), magnesium acetate tetrahydrate (C4H6MgO4·4H2O), and ammonium hydrogen carbonate (NH4HCO3) were analytical reagents purchased from Sinopharm chemical reagent company, Shanghai, and used as raw materials of NMM precursor. The metal salt solution of 1 M was prepared according to its stoichiometric ratio with Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni
Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg = 6
Mg = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Then the NH4HCO3 solution of 1 M (mole ratio: NH4HCO3
1. Then the NH4HCO3 solution of 1 M (mole ratio: NH4HCO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal salt = 1.2
metal salt = 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a precipitating agent was added dropwise into the metal salt solution stirring magnetically 4 h at room temperature to produce the precursor. After the reaction was completed, the precursor was centrifuged and washed with deionized water several times until that the pH value was neutral. The NMM precursors were dried at 60 °C for 10 h and calcined at 900 °C for 1 h.
1) as a precipitating agent was added dropwise into the metal salt solution stirring magnetically 4 h at room temperature to produce the precursor. After the reaction was completed, the precursor was centrifuged and washed with deionized water several times until that the pH value was neutral. The NMM precursors were dried at 60 °C for 10 h and calcined at 900 °C for 1 h.
Fabrication of NMM ceramic microbeads using their ceramic inks
The NMM ceramic inks were composed of the above precursor, the polyvinyl butanol (PVB) as dispersant (0.1–0.3 wt%), the polyethylene glycol 400 (PEG-400) as organic assistant (0.3–0.5 wt%), and the n-pentanol as solvent medium. Appropriate amount of NMM nanoparticles were added to a continuous stirring and ultrasonic above organic solution to form the ceramic inks with the solid content 25 wt%. NMM ceramic inks were very stable and homogeneous over several days. Then NMM nanoceramic microbeads were fabricated by printing about 300 drops of their ceramic inks on the two parallel Pt–Ir wires as electrodes using the ink-jet printer system (Microfab, inkjet II, USA) consisted of a piezoelectric ink-jet head with 80 μm nozzle. The schematic diagram for the preparation of NMM microbeads by inkjet printing are as shown in Fig. 1. After inkjet printing and drying, NMM nanoceramic microbeads were sintered at different TSS process as shown in Fig. 2.
 |
| | Fig. 1 Schematic illustration of preparation of NMM microbeads by inkjet printing. | |
 |
| | Fig. 2 Schematic diagrams of NMM samples via TSS process at different temperatures. | |
Characterization
The crystal phase of NMM powders calcined at 900 °C was recorded by the X-ray powders diffractometer (XRD; Bruker AXS, D2 Advance, Germany) with Cu Kα radiation (λ = 0.15406 nm) in the 2θ range from 20 to 80. Particle sizes of Mn–Co–Ni–O powders were estimated from the XRD patterns in terms of Debye–Scherrer formula (Dhkl = kλ/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ)), where k is a constant, λ is the wavelength, β is the half-width, and θ is the peak angle. SEM images of NMM nanoceramic microbeads at different TSS process were observed with scanning electron microscope (SEM; LEO1430VP, Germany). The electrical resistances of NMM microbeads were measured in an oil bath from 0 °C to 220 °C, in steps of 10 °C, with an Agilent digital multimeter (Agilent Keithley 2400, USA). NMM microbeads were coated a thin film using vacuum coating equipment (LACHI LH300, Taiwan) to make its insulating. NMM insulated microbeads were moved rapidly from room temperature to the uniform ice-water mixtures to change resistance and voltage so as to be recorded using the oscilloscope (Tektronix OPO7354, USA), which could be calculated the time constant of NMM microbeads.
θ)), where k is a constant, λ is the wavelength, β is the half-width, and θ is the peak angle. SEM images of NMM nanoceramic microbeads at different TSS process were observed with scanning electron microscope (SEM; LEO1430VP, Germany). The electrical resistances of NMM microbeads were measured in an oil bath from 0 °C to 220 °C, in steps of 10 °C, with an Agilent digital multimeter (Agilent Keithley 2400, USA). NMM microbeads were coated a thin film using vacuum coating equipment (LACHI LH300, Taiwan) to make its insulating. NMM insulated microbeads were moved rapidly from room temperature to the uniform ice-water mixtures to change resistance and voltage so as to be recorded using the oscilloscope (Tektronix OPO7354, USA), which could be calculated the time constant of NMM microbeads.
Results and discussion
Fig. 3 shows the result of XRD pattern of NMM nanoparticles calcined at 900 °C. According to the XRD pattern of the synthesized NMM powders, the pure NiMn2O4 cubic spinel structure (JCPDS card no. 01-1110) is observed and there are no other diffraction peaks of impurity. The broadened and smooth peaks illustrate that powders are well crystallized. In the NiMn2O4 spinel structure, the large size of Mg2+ (0.072 nm) is substituted for the small size of Mn3+ (0.058 nm) or Mn4+ (0.053 nm) and occupied of sublattices in spinel structure octahedral sites,13 which indicates that the interplanar spacing increases according to the Bragg equation. Therefore, the major diffraction peaks are slightly moved to the small angles, as shown in Fig. 3. Furthermore, the major diffraction peaks at 2θ = 30.132°, 35.510°, 37.141°, 43.146°, 53.426°, 57.091° and 62.614° can be well indexed to the (220), (311), (222), (400), (422), (511) and (224) lattice planes of the cubic spinel structure. Meanwhile, the lattice parameters of the NMM nanopowders are calculated to be 8.3966 Å. The average crystallite size of NMM nanoparticles are estimated about 36 nm according to the Debye–Scherrer formula. In addition, as shown the inset of Fig. 3, NMM nanoparticles are nearly spherical shape with smooth surface, and these powders are very fine and have a narrow and uniform particle size distribution with about 35 nm. The NMM ultrafine powders with high quality have made contribution to the preparation and characterization of NMM ceramic inks and microbeads.
 |
| | Fig. 3 The XRD pattern and SEM image inset of synthesized NMM ceramic nanoparticles calcined at 900 °C. | |
SEM images of NMM nanoceramic microbeads under TSS process at different temperatures are shown as Fig. 4. For NMM samples at TSS process between T1 of 1300 °C and T2 of 1100–1250 °C (Fig. 4a–d), the microstructure strongly depends on the sintering procedures. At the sintering temperature T2 of 1100 °C and 1150 °C, there are a little ceramic grains undeveloped and some pores located at the grain boundaries, while other ceramic grains are fully developed and formed a homogeneous, fine and completely densified microstructure above 1200 °C. The whole NMM ceramic microbeads at sintering temperatures T2 of 1200 °C on average are 140 μm long and well densification as shown in Fig. 4c inset. In addition, as the sintering temperature (T2) of TSS process increases, the grain size of samples increases slightly range from 1.6 to 2.8 μm, as well as the microstructure morphology of ceramics are more regular and dense. It is also indicating that the TSS process can decrease ceramic grain size effectively and increase density. Because the driving force of sintering has used mainly for the densification process not the grain growth for the TSS process. To manage to reach the dense and fine microstructure in the TSS process, proper parameters selection are important particularly.20 In the first step, T1 (1300 °C) of NMM samples should be higher than a critical sintering temperature (1150 °C) of 100 °C to 150 °C so as to rapidly converts the raw nanoparticles into intermediate crystalline state. Due to rapid cooling (10 °C min−1) of NMM samples from T1 to T2, the activation of a triple junction drag mechanism have restrained the grain growth in the second sintering step. A lower T2 and optimal sintering holding time at T2 are necessary to make grain boundary diffusion as well as remove the supercritical pores, which results in the higher densification of NMM ceramic materials. Therefore, setting the optimal TSS process parameters is crucial. In this paper, T1, T2, cooling rate and soaking time of the optimal TSS parameters are 1300 °C, 1200 °C, 10 °C min, and 300 min respectively.
 |
| | Fig. 4 SEM images of the NMM samples via TSS process range from 1100–1250 °C at T2: (a) 1100 °C; (b) 1150 °C; (c) 1200 °C; (d) 1250 °C. | |
The EDS mapping measurement has been also carried out to investigate the element distribution of the Mn, Ni, and Mg elements in the individual NMM microbeads sintered at 1200 °C (Fig. 4c). As shown in Fig. 5, the element mapping image shows that the atomic distributions of the Mn, Ni, and Mg elements are homogeneous mixed in the each NMM ceramic microbead, which further confirms the element composition.
 |
| | Fig. 5 EDS mapping images of NMM microbeads sintered at 1200 °C. | |
Fig. 6 shows the relationship between electrical resistances (R) and absolute temperature (T) for NMM samples. The resistances decrease exponentially as the ambient temperature increases. The relationships between ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R and the reciprocal of absolute temperature (1/T) for NMM nanoceramic microbeads are shown in Fig. 7. It can be observed that these linear relationships of NMM samples are exhibited the well negative temperature coefficient thermistor characteristics, which is described by the Nernst–Einstein relationship.
R and the reciprocal of absolute temperature (1/T) for NMM nanoceramic microbeads are shown in Fig. 7. It can be observed that these linear relationships of NMM samples are exhibited the well negative temperature coefficient thermistor characteristics, which is described by the Nernst–Einstein relationship.
| |
 | (1) |
with
| |
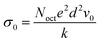 | (2) |
where
Noct is the concentration of the octahedral sites per cubic centimeter,
d is the jump distance for the charge carriers,
v0 is the lattice vibrational frequency,
k is the Boltzmann constant,
N is the concentration per formula unit of sites which is available to the charge carriers, and
EH is the hopping energy. In addition, the number of charge carriers
NC(1 −
C) is related to the number of M
n+ and M
(n+1)+ (M = Mn or Ni) cations located on the octahedral sites of spinel structure. N is the number of total charge carriers and
C(1 −
C) is the probability of finding one donor and one acceptor on two adjacent identical sites. The resistances at 25 °C (
R25), thermal constant (
B25/100), temperature coefficient of resistance (
α25), the activation energy (
Ea) and the slopes of ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R versus
R versus (1/
T) plots as shown in
Fig. 7 for NMM samples are listed in
Table 1 to illustrate the electrical properties variation exactly. These parameters are calculated by
eqn (3)–(5).
18,19,23| |
 | (3) |
| |
 | (5) |
where
R25 and
R100 are the resistances measured at 25 °C and 100 °C, respectively. The slopes of ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R versus
R versus 1/
T plots, as shown in
Fig. 7, can be regarded as an assessment of the activation energy (
Ea) and the coefficient of temperature sensitivity. As shown in
Table 1, the values of
R25,
B25/100,
Ea,
α25 and the slope for the NMM samples sintered range from 1100 °C to 1250 °C are the range of 98
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
331–174
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
601 Ω, 4176–4359 K, 0.364–0.379 eV, −4.8 to 4.9%/K, and 4.25–4.44, respectively.
 |
| | Fig. 6 Relationship between R and T of NMM ceramic microbeads. | |
 |
| | Fig. 7 Relationship between ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R and (1000/T) of NMM ceramic microbeads. R and (1000/T) of NMM ceramic microbeads. | |
Table 1 The resistivity at 25 °C (R25), thermistor resistance value (B25/100), temperature coefficient of resistance (α25), slope, activation energy (Ea) and time constant of NMM samples
| Samples |
R25 (Ω) |
B25/100 (K) |
Ea (eV) |
Slope (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R − 1/T) R − 1/T) |
α25 (%/K) |
τ (ms) |
| 1100 |
174![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 601 601 |
4390 |
0.379 |
4.44 |
−4.9 |
147 |
| 1150 |
153![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 523 523 |
4332 |
0.374 |
4.43 |
−4.9 |
99 |
| 1200 |
126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 969 969 |
4225 |
0.364 |
4.25 |
−4.8 |
72 |
| 1250 |
98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 331 331 |
4183 |
0.365 |
4.27 |
−4.8 |
58 |
As shown in Fig. 6 and 7, the resistances R25, and B constant of MCN ceramic samples are significantly dependent on the different sintering temperatures. As the lower sintering temperature (T2) of TSS process increases from 1100 to 1250 °C, the room temperature resistances R25, and the B value decrease when the higher sintering temperature (T1) is constant. The electrical properties of the NMM ceramic samples are mainly dependent on the crystal structure, chemical composition, the microbead body size and sintering process.5,17 NMM ceramic inks are inkjet-printed quantitatively and accurately onto these wires using the same batch of ceramic inks by drop-on-demand ink-jet printer system. As shown above, the whole synthesized NMM samples have the same chemical composition, microbead body size and crystal structure. Hence, the sintering process plays an important role in their electrical properties. This could be explained as follows: in the TSS process, for the NMM microbeads, the grain size increases and the pores decrease with the sintering temperature (T2) increasing. The densification improved of NMM microbeads can effectively increase the hopping probability of the electron scatting events among the charge carriers, resulting in the grain boundary resistance and the B constant decreasing.
Fig. 8 shows the signal V response changed by the temperature range from 0 to 25 °C of NMM microbeads samples. The signal V response increases with the temperature decreases because of the well negative temperature coefficient characteristics of NMM samples. Thermal time constant of NMM samples is the 63.2% time needed from the initial temperature varying to the final temperature, when the ambient temperature change abruptly. As shown in Fig. 8, it is calculated that thermal time constant of NMM samples is about 58 ms by eqn (6) and (7):24,25
| |
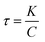 | (7) |
where
P is the power dissipated in the resistance samples (
V being the voltage across the samples, and
I the constant current about 100 μA),
T is the sample temperature and
T0 the ambient temperature.
K is the dissipation constant of NMM resistance samples, and the
C is the thermal capacity of NMM samples.
 |
| | Fig. 8 Temperature-controlled waveforms of the NMM microbeads coated thin film. | |
The measurement of dissipation constant K is based on the experimentally determined R(T) characteristics and on the measurement of NMM sample resistance for several values of applied electrical power. For instance, in the case of negative temperature coefficient thermistors, NMM samples, the R(T) characteristic under conditions of not negligible dissipation is of the form:26
| |
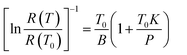 | (8) |
According to the above equation, it is indicating that the thermal time constant is proportional to the input power P, and the P is proportional to the resistance R of NMM samples, i.e. the thermal time constant decreases as the input power and the resistance of samples decreases. As shown in Fig. 9, with the sintering temperature increasing, the resistance of NMM samples decreases, thus the thermal time constant decreases from 58 to 147 ms.
 |
| | Fig. 9 Time constant and B constant of NMM samples as a function of sintering temperature. | |
Conclusion
This study demonstrates that high dense NMM nanoceramic microbeads have been fabricated via inkjet printing using their ceramic inks with prominent stability, dispersivity and homogeneity. NMM nanoparticles synthesized via co-precipitation method have shown well-defined single cubic spinel NMM nanocrystallines with particle size about 36 nm. Furthermore, NMM nanoceramic microbeads sintered by the TSS process using optimal sintering parameters, have exhibited the high dense microstructure and well electrical properties at T1 of 1300 °C and T2 of 1250 °C. NMM microbeads have been achieved with moderate resistance, lower thermal time constant and higher material constant B for the first time. The successful breakthrough of high performance of NMM nanoceramic microbeads ensures the opportunity for a broader range of applications.
Acknowledgements
This work was supported by the National High Technology Research and Development Program of China (Grant No. 2012AA091102), the One Hundred Talent Program of Chinese Academy of Sciences, Chinese Academy of Sciences (Grant No. YZ201261), the West Light Foundation of Chinese Academy of Science (No. XBBS201314) and the Talent Program of Xinjiang Uygur Autonomous Region.
References
- P. Calvert, Chem. Mater., 2001, 13, 3299–3305 CrossRef CAS.
- H. Sirringhaus, T. Kawase, R. H. Friend, T. Shimoda, M. Inbasekaran, W. Wu and E. P. Woo, Science, 2000, 290, 2123–2126 CrossRef CAS PubMed.
- H. Minemawari, T. Yamadal, H. Matsui, J. Tsutsumi, S. Haas, R. Chibal, R. Kumai and T. Hasegawa, Nature, 2011, 475, 364–367 CrossRef CAS PubMed.
- B. J. de Gans, P. C. Duineveld and U. S. Schubert, Adv. Mater., 2004, 16, 203–213 CrossRef CAS.
- A. Kamyshny and S. Magdassi, Small, 2014, 10, 3515–3535 CrossRef CAS PubMed.
- A. J. Nozik, M. C. Beard, J. M. Luther, M. Law, R. J. Ellingson and J. C. Johnson, Chem. Rev., 2010, 110, 6873–6890 CrossRef CAS PubMed.
- B. G. Levi, Phys. Today, 2001, 54, 20 Search PubMed.
- S. Madhusudan, H. M. Hanna and D. Parul, Adv. Mater., 2010, 22, 673–685 CrossRef PubMed.
- X. Liu, T. J. Tarn, F. F. Huang and J. Fan, Particuology, 2015, 19, 1–13 CrossRef CAS.
- B. Derby, J. Eur. Ceram. Soc., 2011, 31, 2543–2550 CrossRef CAS.
- X. Luo, Z. G. Zeng and X. H. Wang, J. Power Sources, 2014, 271, 174–179 CrossRef CAS.
- S. J. Joerg, H. C. Rudolf and E. Joerg, J. Mater. Chem., 2009, 19, 1449–1457 RSC.
- M. J. van den Berg Antje, W. M. de laat Antonius and J. S. Patrick, J. Mater. Chem., 2007, 17, 671–683 Search PubMed.
- T. Kaydanova, A. Miedaner and J. D. Perkins, Thin Solid Films, 2007, 515, 3820–3824 CrossRef CAS.
- J. C. Yao, B. Y. Zhang, J. H. Wang and A. M. Chang, et al., Mater. Lett., 2013, 112, 69–71 CrossRef CAS.
- C. W. Peng, H. M. Zhang and A. M. Chang, et al., J. Mater. Sci.: Mater. Electron., 2012, 23, 851–857 CrossRef CAS.
- S. A. Kanade and V. Puri, Mater. Lett., 2006, 60, 1428–1431 CrossRef CAS.
- J. B. Xia, Q. Zhao and A. M. Chang, J. Alloys Compd., 2015, 646, 249–256 CrossRef CAS.
- K. Park, S. J. Kim, J. G. Kim and S. Nahm, J. Eur. Ceram. Soc., 2007, 27, 2009–2016 CrossRef CAS.
- I. W. Chen and X. H. Wang, Nature, 2000, 404, 168–171 CrossRef CAS PubMed.
- B. Katarina, G. Dusan and S. Peter, Ceram. Int., 2015, 41, 11975–11983 CrossRef.
- Y. I. Lee, Y. W. Kim and M. Mitomo, J. Am. Ceram. Soc., 2003, 86, 1803–1805 CrossRef CAS.
- K. Park and I. H. Han, J. Electroceram., 2006, 17, 1069–1073 CrossRef CAS.
- M. Kimura and K. Toshima, Sens. Actuators, A, 2003, 108, 239–243 CrossRef CAS.
- T. Shuilin, C. L. Fred and M. Paolo, IEEE Transactions on Power Electronics, 2014, 29, 4450–4460 CrossRef.
- Z. M. Huang, W. Zhou and O. Y. Cheng, et al., Sci. Rep., 2015, 5, 10899 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26760a |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 331–174
331–174![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 601 Ω), lower thermal time constant (58–147 ms), and higher material constant (4176–4309 K) are achieved by tuning the sintering temperatures.
601 Ω), lower thermal time constant (58–147 ms), and higher material constant (4176–4309 K) are achieved by tuning the sintering temperatures.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni
Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg = 6
Mg = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Then the NH4HCO3 solution of 1 M (mole ratio: NH4HCO3
1. Then the NH4HCO3 solution of 1 M (mole ratio: NH4HCO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal salt = 1.2
metal salt = 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a precipitating agent was added dropwise into the metal salt solution stirring magnetically 4 h at room temperature to produce the precursor. After the reaction was completed, the precursor was centrifuged and washed with deionized water several times until that the pH value was neutral. The NMM precursors were dried at 60 °C for 10 h and calcined at 900 °C for 1 h.
1) as a precipitating agent was added dropwise into the metal salt solution stirring magnetically 4 h at room temperature to produce the precursor. After the reaction was completed, the precursor was centrifuged and washed with deionized water several times until that the pH value was neutral. The NMM precursors were dried at 60 °C for 10 h and calcined at 900 °C for 1 h.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ)), where k is a constant, λ is the wavelength, β is the half-width, and θ is the peak angle. SEM images of NMM nanoceramic microbeads at different TSS process were observed with scanning electron microscope (SEM; LEO1430VP, Germany). The electrical resistances of NMM microbeads were measured in an oil bath from 0 °C to 220 °C, in steps of 10 °C, with an Agilent digital multimeter (Agilent Keithley 2400, USA). NMM microbeads were coated a thin film using vacuum coating equipment (LACHI LH300, Taiwan) to make its insulating. NMM insulated microbeads were moved rapidly from room temperature to the uniform ice-water mixtures to change resistance and voltage so as to be recorded using the oscilloscope (Tektronix OPO7354, USA), which could be calculated the time constant of NMM microbeads.
θ)), where k is a constant, λ is the wavelength, β is the half-width, and θ is the peak angle. SEM images of NMM nanoceramic microbeads at different TSS process were observed with scanning electron microscope (SEM; LEO1430VP, Germany). The electrical resistances of NMM microbeads were measured in an oil bath from 0 °C to 220 °C, in steps of 10 °C, with an Agilent digital multimeter (Agilent Keithley 2400, USA). NMM microbeads were coated a thin film using vacuum coating equipment (LACHI LH300, Taiwan) to make its insulating. NMM insulated microbeads were moved rapidly from room temperature to the uniform ice-water mixtures to change resistance and voltage so as to be recorded using the oscilloscope (Tektronix OPO7354, USA), which could be calculated the time constant of NMM microbeads.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R and the reciprocal of absolute temperature (1/T) for NMM nanoceramic microbeads are shown in Fig. 7. It can be observed that these linear relationships of NMM samples are exhibited the well negative temperature coefficient thermistor characteristics, which is described by the Nernst–Einstein relationship.
R and the reciprocal of absolute temperature (1/T) for NMM nanoceramic microbeads are shown in Fig. 7. It can be observed that these linear relationships of NMM samples are exhibited the well negative temperature coefficient thermistor characteristics, which is described by the Nernst–Einstein relationship.
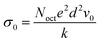
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R versus (1/T) plots as shown in Fig. 7 for NMM samples are listed in Table 1 to illustrate the electrical properties variation exactly. These parameters are calculated by eqn (3)–(5).18,19,23
R versus (1/T) plots as shown in Fig. 7 for NMM samples are listed in Table 1 to illustrate the electrical properties variation exactly. These parameters are calculated by eqn (3)–(5).18,19,23

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R versus 1/T plots, as shown in Fig. 7, can be regarded as an assessment of the activation energy (Ea) and the coefficient of temperature sensitivity. As shown in Table 1, the values of R25, B25/100, Ea, α25 and the slope for the NMM samples sintered range from 1100 °C to 1250 °C are the range of 98
R versus 1/T plots, as shown in Fig. 7, can be regarded as an assessment of the activation energy (Ea) and the coefficient of temperature sensitivity. As shown in Table 1, the values of R25, B25/100, Ea, α25 and the slope for the NMM samples sintered range from 1100 °C to 1250 °C are the range of 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 331–174
331–174![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 601 Ω, 4176–4359 K, 0.364–0.379 eV, −4.8 to 4.9%/K, and 4.25–4.44, respectively.
601 Ω, 4176–4359 K, 0.364–0.379 eV, −4.8 to 4.9%/K, and 4.25–4.44, respectively.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R − 1/T)
R − 1/T)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 601
601![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 523
523![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 969
969![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 331
331