FePt nanoparticles: a novel nanoprobe for enhanced HeLa cells sensitivity to chemoradiotherapy
Abstract
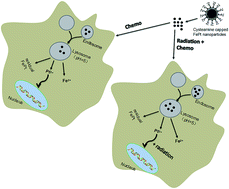
Chemoradiotherapy is a well-established treatment paradigm in oncology to consistently improve local tumor control compared to the sole administration of chemotherapy or radiotherapy. Despite its importance, few agents have been identified to further improve the therapeutic ratio and reduce the incidence of complications. Advances in nanomedicine have offered innovative strategies to improve chemoradiotherapy. In the present study, we evaluated the efficacy and safety of FePt nanoparticles (NPs) in chemoradiotherapy using HeLa cells and HEK293T cells. FexPt100−x NPs at different compositions (x = 26, 53 and 77) were synthesized and characterized by means of X-ray diffraction, transmission electron microscopy, and Fourier transform infrared spectroscopy. After ligand exchange, the cytotoxicity of FexPt100−x NPs was evaluated by MTT assay in HEK293T cells while the composition effects of FePt NPs on the cytotoxicity and the potential application of FePt NPs in combination with X-ray radiation at clinically relevant MV energies were investigated in HeLa cells in vitro. Besides, the cellular uptake of NPs was measured indirectly by atomic absorption spectroscopy and transmission electron microscopy. The results indicated that FexPt100−x NPs inhibited the growth of HeLa cells in a concentration- and composition-dependent manner after 24 h incubation, with low cytotoxicity to HEK293T cells at the given concentrations (0–20 μg mL−1). Furthermore, the combination of FePt NPs and radiotherapy resulted in a marked inhibition of HeLa cells, in contrast with that of the individual FePt NPs treated group or the radiation alone group. Moreover, Fe53Pt47 NPs, exhibiting significant cytotoxicity and enhanced radiosensitization effects on HeLa cells without damage to HEK293T cells, might theoretically satisfy the ultimate goal of personalized chemoradiotherapy. Our present work exhibited high therapeutic efficacy of FePt NPs in combination with radiotherapy without apparent cytotoxicity, suggesting the potential of FePt NPs as a promising nanoprobe in improving the outcome of tumor chemoradiotherapy.

 Please wait while we load your content...
Please wait while we load your content...