Exploring the structure and stability of amino acids and glycine peptides in biocompatible ionic liquids
Awanish Kumar
,
Meena Bisht
and
Pannuru Venkatesu
*
Department of Chemistry, University of Delhi, Delhi-110 007, India. E-mail: venkatesup@hotmail.com; pvenkatesu@chemistry.du.ac.in; Fax: +91-11-2766 6605; Tel: +91-11-27666646-142
First published on 1st February 2016
Abstract
Amino acids (AAs) are vital components for a variety of biological systems and can be linked through covalent bonds (or peptide bonds) to form a protein structure. Essentially, the interactions of these AAs with solvents as well as co-solvents/solutes determine the thermodynamic stability of the protein. In this context, this review represents an overview of the current status of the thermodynamic effect of ionic liquids (ILs) on AAs and glycine peptides (GPs). Moreover, ILs are considered as green solvents for many chemical and biological processes due to their tunable physical properties. Interestingly, these ILs can adjust themselves in any required experimental conditions such as protein extraction to enzyme catalysis. In this review, we attempt to assess the status of our current understanding on the biocompatible nature of the ions of ILs on AAs and protein model compounds and their functional groups with some precisely available experimental data in the literature. Examples of current applications of the ILs on protein model compounds are also covered in this review.
1. Introduction
The basic structural unit of proteins are the amino acids (AAs),1,2 which play a two-fold role both as building blocks of proteins and as intermediates in metabolism. Determination of the solvation properties of AAs in various solvents such as water and ionic liquids (ILs) enables us to understand protein structure and its stability in solvent systems. This is biologically important since AAs can be used for commercial applications in human foods and in pharmaceuticals. They are also used as intermediates in the synthesis of a variety of biomolecules. The interaction between solvent and the various constituent groups of a protein, such as the AA side-chains, is essentially responsible for the protein stability. Also a combination of these AAs generates a peptide backbone unit (–CH2CO⋯NH–) that also plays a central role in the structural stabilities of proteins in aqueous medium.3–13 The analysis of the thermodynamic properties such as transfer free energy (ΔG′tr) of the AAs provides set of data that can predict the stability of proteins in aqueous medium with various co-solvents or solutes as additives. This underlying principle was successfully introduced and implemented by Kauzmann14 using the thermodynamic data for model compounds in elucidating the interactions contributing to protein stability. A more quantitative analysis was performed by Tanford,15 who accepted that analysis of the thermodynamic contribution of individual AAs and similar model compounds can accurately predict the stability of the proteins.In recent years, due to new environmental regulations, the challenge of using environmental-friendly solvents has prompted a great development of innovative products to protect the environment. The awareness regarding the impact of the chemicals on environment is increasing globally. In this context, ILs, have been considered as the replacement for organic solvents to reduce the environmental contamination.16–19 The excellent physicochemical properties and unique biodegradability of ILs can be regarded as an attractive replacement for organic solvents.19–21 Therefore, ILs are receiving a very good response from the industrial community.22–26 Hence, ILs have the potential to fulfill the requirement of environment sustainability.
Interestingly, biocompatibility has been achieved for a number of these ILs in the recent years.27–32 Stabilization, activity, and structure of amino acids (AAs) and protein in ILs have been reported by our research group on several occassions.27–46 However, up to now, no reliable review exists to predict the current understanding on the nature of interactions between the AAs and the ILs. This review covers the results of the investigations on the properties and behavior of the AAs and GPs in various ILs aqueous solutions. This kind of approach will allows us to draw conclusions concerning the principles responsible for the stability of proteins in ILs.
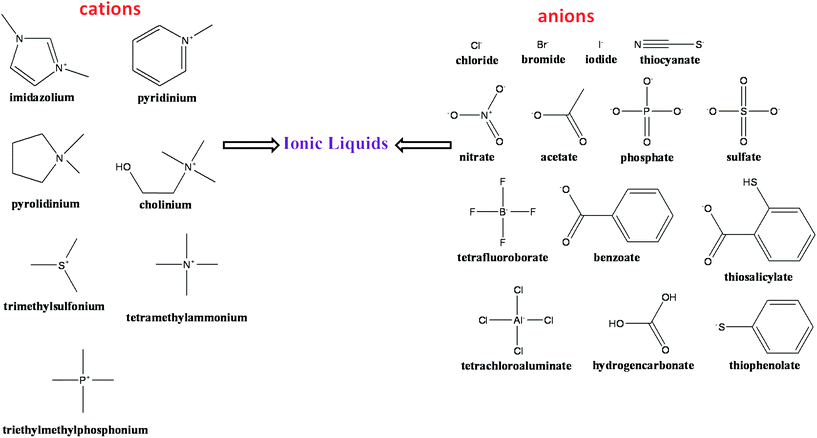
2. A layout for ionic liquids (ILs)
The term ILs describe a popular class of organic salts that melt below ∼100 °C and have an appreciable liquid range.47–54 ILs typically consist of organic nitrogen-containing heterocyclic cations and inorganic anions.51–53 An excellent short history of the birth of ILs is presented by John S. Wilkes.53 Common ILs includes ammonium, phosphonium, sulfonium, guanidinium, pyridinium, imidazolium, and pyrrolidinium cations. The most common anions are chloride, bromide, tetrafluoroborate, hexafluorophosphate, trifluoromethanesulfonyl, bis(trifluoromethanesulfonyl)imide, dicyanamide and alkylsulfate anions. Fig. 1 represents a selection of some common cations and anions used in the preparation of ILs. ILs have an advantage of a negligible vapor pressure that make them a potential candidates for industrial applications and a least harmful air pollutant.50,51,55Apparently, ILs can lessen the risk of air pollution due to their insignificant vapor pressure, and low toxicity of ILs. Very recently, Egorova and Ananikov56 showed that the biological activity and cytotoxicity of ILs dramatically depend on the nature of a biological system. They concluded that an IL may not be toxic for particular cells or organisms, but may demonstrate high toxicity towards another target present in the environment. Therefore, it seems that the interactions of the ILs with the biological systems are specific and it has been further proven that a stabilizing IL for a particular protein does not necessarily act as stabilizer for another protein.48 Furthermore, Petkovic et al.57 presented an overview of the current understanding of the toxicity of the ILs and its impact on the environment. Moniruzzaman et al.58 compared ILs with conventional organic solvents in enzymatic reactions and concluded that use of enzymes in ILs has presented many advantages such as high conversion rates, high enantioselectivity as well as better recoverability and recyclability as compared to the conventional organic solvents. ILs are often being classified as green solvent because of their high surface activity and low aqueous toxicity. Furthermore, the effects of ILs on the enantioselectivity of biocatalytic transformations in comparison with conventional reaction media have been reviewed by van Rantwijk and Sheldon.59 Therefore, ILs are now considered widely as a much greener solvent and replacement for the volatile organic compounds. Based on open literatures, ILs reduce both environmental pollution and economy. Thereby these ionic liquids display a wide range of potential application as greener solvents for all scientific communities (Scheme 1).
 | ||
| Scheme 1 The schematic depiction of the ILs as environment friendly solvents in various scientific fields. | ||
3. Thermodynamic contribution of amino acids (AAs) in ILs towards protein stability
Different AAs can be linked through the covalent bonds (or peptide bonds) to form a linear chain of polymeric structure called the protein. Thousands of possible modes of arrangement of AAs in a chain can be obtained but only a unique sequence of AAs is functional.60,61 Protein folding is a complicated process, hence, simple model peptides as well as AAs are usually used to understand the functions of the proteins with the use of thermodynamic properties (Scheme 2).62 The seminal denaturation/renaturation experiments performed by Anfinsen62 indicate that the protein folding is contained entirely in the AA sequence of the protein. Computer-based simulation experiments seemed to corroborate this conclusion.63 However, despite noticeable progress in the field of protein stabilities and the availability of advanced techniques and authoritative computation, the protein folding laws still remains ambiguous.Moreover, successful attempts have been made in mimicking the functionally active protein sequences at a laboratory level which are very much beneficial therapeutically.64,65 However, stabilizing a sequence in various media is still a thermodynamic problem. Guzmán et al.66 put forward the real drawbacks in peptide stability in synthesis procedures and illustrated that in the past few years, more than 250 protecting groups have been proposed suitable for peptide synthesis; however, a relatively small number of those is actually used because of the stringent requirements that a protecting group should meet, particularly with respect to the requirement of the preservation of other functionalities.
The search of a new solvent medium in order to improve the solubility and stability of peptides and proteins in solutions during synthesis, and their chemical characterization has occupied the mind of peptide chemists. During the past three years, in this mission of extensive researches, interest in the use of ILs in peptide and protein research has immensely increased.67–69 Plaquevent et al.67 reviewed the beneficial aspects and outcomes of using ILs as a new media that could be particularly suitable for AAs and peptide synthesis since the polarity of ILs provides much ideal environment for such experiments. Hence, a detailed study is essentially required including the investigations on the influence of the ILs on the AA structures.
4. Thermodynamics of AA stability: the concept of apparent transfer free energy (ΔG′tr)
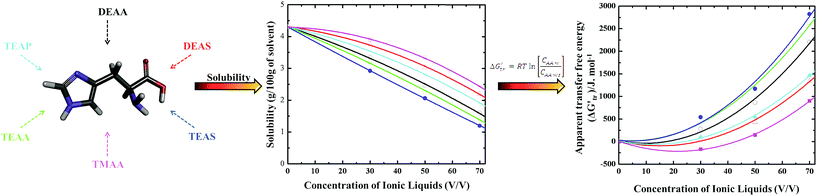
The solvent–protein interactions comprehend the profound influence of the solvent on AAs in the protein structure. Aqueous solutions provide best medium for biomolecules functioning and hence it is clear that water can influence protein conformation and stability by competing for hydrogen-bonding sites of the biomolecule and effectively solvating the polar AAs. The protein–water hydrogen bonds might then compete efficiently with the intra-molecular hydrogen bonds and easily break the folded state in the presence of the co-solvents.70–73 Therefore, the interactions between co-solvents and AAs in an aqueous environment have been studied by the transfer free energy (ΔG′tr). The term ΔG′tr was introduced in 1959 by Kauzmann14 that elucidated the interactions contributing to the protein model compound stability in co-solvents.Later, a more quantitative analysis was exploited by Nozaki and Tanford74–76 who accepted the paradigm of transfer between two phases and estimated the hydrophobic contributions to the stability of AAs. Apparent transfer free energy ΔG′tr is obtained through the solubility of AAs in water-co-solvent mixture which is schematically shown in Scheme 3. Nozaki and Tanford74–76 delineated that the ΔG′tr of a given protein is simply the sum of the ΔG′tr of the solvent exposed AA residues. Based on the solubility measurements of small AAs and peptides in an aqueous-co-solvent mixture, the ΔG′tr of individual AAs can be obtained and thermodynamic hypothesis with the group transfer model.3,4,11 A basic level of quantifying protein–solvent interactions involves the use of ΔG′tr. The ΔG′tr of AAs from water to a co-solvent system will be either favorable or unfavorable. By definition, a value of ΔG′tr > 0, means an unfavorable interaction of AAs with the co-solvent i.e., the AA is less soluble on transfer to a co-solvent from pure aqueous medium, which indirectly accounts for an increase in AA stability in the co-solvent.3,4 On the other hand, a negative value ΔG′tr < 0, indicates that AA ΔG′tr becomes more favorable to the co-solvent and therefore AA is more soluble in aqueous co-solvent solution, which indicates the destabilization of protein model compounds.3,4
 | ||
| Scheme 3 The schematic description obtaining of apparent transfer free energy (ΔG′tr) through the solubility of AAs in water or ILs. | ||
5. Interactions between model protein compound and ILs
The direct study on the stability of individual AAs in ILs make no sense, rather, it is defined in the terms of electrostatic or hydrophobic interactions of the various groups of AAs with the constituent ions of ILs.77 In this regard, ILs have been significantly used in biphasic mixtures to extract the AAs from the solution mixture.78,79 Based on the data of the extent of extractions of AAs in ILs, majority of the imidazolium-based ILs have been used to investigate protein stability.80–82 However, the basic level of investigation of the AAs interactions with ions of IL is very rare.83–85 Moreover, attempts have been performed to separate the AAs from aqueous media using IL–water aqueous biphasic systems. Furthermore, the use of AAs as phase separating agents has been widely considered.86 One of the most important properties of such two-phase systems is the mutual solubility of the IL and water that has been considered as the biocompatible media for biological molecules such as AAs and proteins.69,87 The aqueous systems are based on the solubility behavior of an IL in water and the electrochemical properties at the interface between the two phases are intimately related with each other.88 Interestingly, two-phase systems formed by a hydrophobic IL and water find several useful applications in separation process. In this context, biphasic mixtures of water and hydrophobic ILs such as 1-hexyl-3-methylimidazolium tetrafluoroboarte ([Hexmim][BF4]) and 1-decyl-3-methylimidazolium tetrafluoroborate ([Dmim][BF4]) have been significantly used by Rodriguez et al.89 to extract AAs such as alanine (Ala), valine (Val), leucine (Leu) and α-amino-n-caprylic acid. Their experiment demonstrated that the AAs distribute preferentially to the IL phase, which increases with the length of the AA side chain. Alternatively, Wang et al.90 observed that, imidazolium-based ILs such as 1-butyl-3-methylimidazolium hexafluorophosphate ([Bmim][PF6]), 1-hexyl-3-methylimidazolium hexafluorophosphate ([Hexmim][PF6]), [Hexmim][BF4] and [Dmim][BF4] can be used as alternative solvents for the recovery of some AAs such as tryptophan (Trp), phenylalanine (Phe), tyrosine (Tyr), Leu and Val from aqueous media. The extraction degree of the AAs decreases with increasing length of alkyl substituent chain on the cation of the ILs, but it increases with increasing solubility of water in IL phases.90 This indicates the importance of the hydrophobic effect as a driving force for the extraction of AAs in ILs.Imidazolium-based ILs aqueous two-phase systems have been uniformly used to investigate their role in separating AAs from the reaction mixture. Especially, the extraction of Trp has been considered in most of the studies. Trp has long been known to be sensitive to the polarity of its local environment that directly reflects the interactions of the solute/solvent or co-solvents with the protein surface or with the Trp itself. The AA consists of the polar amine and carboxylic groups with a non-polar indole side chain. Undoubtedly, the Trp fluorescence wavelength is widely used as a tool to monitor changes in proteins and to make inferences regarding local structure and dynamics.91
It is now evident that the cation hydrophobicity is the major factor that contributes to the extraction of the AAs from the aqueous phase. The effect of anions also adds some contribution to partition effects of AAs. To gain an insight into the driving forces of AA partitioning in the studied IL-based aqueous biphasic systems (ABS), Moattar and Hamzehzadeh92 investigated the distribution of five model AAs such as Trp, Phe, Tyr, Leu, and Val in aqueous medium at different pH values. Their studies indicated that hydrophobic interactions were the main driving force, although electrostatic interactions and salting-out effects were also important for the transfer of the AAs. The importance of anionic nature and the alkyl chain length on the cation of ILs have also been examined. It is noted that IL with BF4− anion has much higher extraction efficiency for the AAs than those with PF6− anion because of the stronger effective charge in BF4−.90,92 Similarly, Absalan et al.93 proposed that the efficiency of the extraction of the AAs in imidazolium-based ILs containing BF4− anions was significantly increased. Accordingly, they investigated the partitioning behavior of acidic, basic and neutral AAs into imidazolium-based ILs from the aqueous phase. In their work,93 they observed that the partitioning behavior of Ala (neutral), Glutamic acid (Glu) (acidic) and lysine (Lys) (basic) AAs was increased in the [Hexmim][BF4] as compared to 1-octyl-3-methylimidazolium tetrafluoroborate ([Omim][BF4]).93 These observations point towards the importance and role of anions in the ILs and their influence on the IL-mediated separation of various AAs. The enhancement of the partitioning of the AAs in BF4− imidazolium-based ILs over PF6− imidazolium-based ILs has been explained on the basis of the effects of water on the structural properties of imidazolium-based ILs. Cammarata et al.94 reported that in the presence of significant amount of water, the anions of the imidazolium-based ILs are completely dissociated and are H-bonded with the water molecules. FTIR results illustrate that the water content in ILs and the strength of hydrogen bonds between water molecules and the anions of ILs follow the order BF4− > PF6−.94 Therefore, it can be considered that the number of free BF4− ions and its ionic mobility in the IL phase is enhanced than the PF6− containing imidazolium-based ILs. This increase would then enhance the electrostatic interactions between the AAs and the BF4− of the IL significantly leading to the partitioning of the AAs in an IL with higher water solubility.
Moreover, the charge density of BF4− is calculated to be higher than the PF6− anions for imidazolium-based ILs.93 Furthermore, quantum chemical calculations also support that the effective negative charge in BF4− is much more than that in PF6−.95 According to Tsunekawa et al.95 in BF4− ion, negative charge is higher in fluorine (F) atoms in a tight space, whereas in PF6− comparatively little negative charge spreads into the atoms in a comparatively wide space. Thus, a BF4− ion can strongly attract a charged AA, as a result in easier formation of a contact ion-pair thus facilitating the separation of the AAs from the aqueous phase.
The interactions between the AAs and ILs are not completely dependent on the ions of the ILs that pose specific interaction with the AA. Rather, it is also favored by the electrostatic charge at the AAs in the specified solvent environments. Working with a set of acidic, basic and neutral AAs such as Ala, Glu, and Lys, Absalan et al.93 found that electrostatic interactions of cationic and anionic sites of ILs with anionic and cationic forms of AAs, respectively, resulted in partitioning of AAs into IL phase. The experimental results pointed towards noticeable electrostatic interactions of anionic part of IL's with cationic forms of AAs that were stronger than electrostatic interactions of cationic part of IL's with anionic forms of AAs which relatively caused a higher partitioning of cationic forms of AAs into IL phase. Additionally, Dreyer and Kragl96 stated that the electrostatic interactions between charged AA residues on the protein's surface and the ions of the IL are the driving forces for the extraction of a biomolecule in IL–water biphasic systems.
From the above experimental work, it appears that the partitioning of AAs in ABS systems depends not only on the physical properties of the components forming the ABS system (in most of the cases water/IL ABS) but also on the properties of the AA in both the components. Selective interaction of AA with any one of the component in the ABS, increases the partitioning of the AA in a particular phase. Compared with hydrophilic ILs, hydrophobic ILs are more accessible to the partition of the AA from the aqueous phase, however, only a limited number of these ILs have been commonly used in partitioning studies.
Our group has studied the of AAs glycine (Gly), Ala, Val,Trp, Tyr, Histidine (His), Leu, theronine (Thr), glutamine (Gln) and serine (Ser), glycine peptides (GPs) (diglycine (Gly2), triglycine (Gly3), tetraglycine (Gly4)) and cyclic dipeptides (CDs) cyclic glycyl–glycine (c(Gly–Gly)), cyclic alanine–glycine (c(Ala–Gly)), cyclic alanine–alanine (c(Ala–Ala)), cyclic leucine–alanine (c(Leu–Ala)), and cyclic valine–valine (c(Val–Val)) in ammonium-based ILs such as diethyl ammonium acetate ([Et2NH][CH3COO], DEAA), diethyl ammonium sulfate ([Et2NH][HSO4], DEAS), triethyl ammonium acetate ([Et3NH][CH3COO], TEAA), triethyl ammonium sulfate ([Et3NH][HSO4], TEAS), triethyl ammonium phosphate ([Et3NH][H2PO4], TEAP) and trimethyl ammonium acetate ([Me3NH][CH3COO]), TMAA.36–41,45 A complete overview of the interactions of model compounds of proteins is provided in our recent review.97 However, here we only present the outline of the findings.
Fig. 2–4 display the results of the for the various AAs, GPs and CDs, respectively, in different ammonium-based ILs (50% (v/v)) at 25 °C. A close analysis of the results in these figures illustrate that the thermodynamic property is dependent on the solubility as well as the individual interactions of each AAs with the IL. Fig. 2 represents the for the AAs in the ILs such as DEAA, DEAS, TEAA, TEAS, TEAP, and TMAA. As it is evident that the is positive for the AAs in all studied ILs except Phe, Thr and Gln in the presence of DEAS and TEAS, in which, the negative trend in is observed. Furthermore, Phe in TMAA only shows the negative trend in the values. An overall comparison between the ILs reveals that the sulfate-based ILs such as DEAS and TEAS are disturbing the structures of Phe, Thr and Gln, since they impart negative. Therefore, DEAS and TEAS are not biocompatible ILs for the structures of Phe, Thr and Gln while the same ILs are biocompatible for the rest of the studied AAs. Apparently, there is no consistency in predicting DEAS as a universal non-biocompatible IL. In this context, Attri and Venkatesu32 observed that IL DEAS is a weak stabilizer for native structure of the α-chymotrypsin (CT) among the studied ammonium-based ILs. In other words, the same authors explored that sulfate-based ammonium family IL was a good stabilizing agent for the native structure of myoglobin (Mb).42
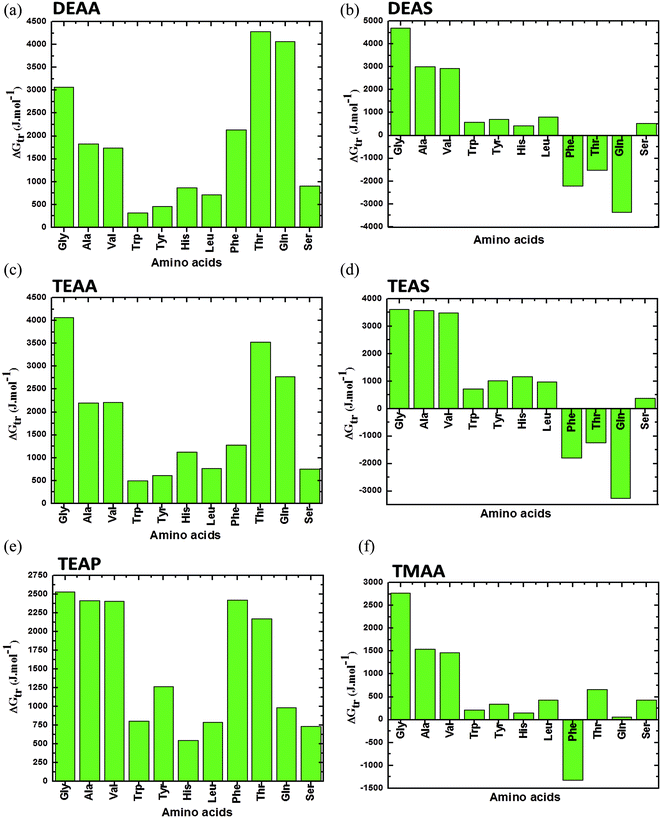 | ||
| Fig. 2 Apparent transfer free energies (ΔG′tr) of AAs from water to ammonium-based IL (50% (v/v)) solution at 25 °C (a) DEAA; (b) DEAS; (c) TEAA; (d) TEAS; (e) TEAP; (f) TMAA (data points are collected from ref. 36, 37 and 40). | ||
Moreover, a comparison between the AAs in each panel of Fig. 2 reveals that AAs such as Trp, Tyr, Leu and His have the lowest values of ΔG′tr, which indicates that these AAs in ILs are vulnerable to the direct unfavorable interactions with the ions of the ILs. And it is well documented throughout the literature that the major cause of the protein denaturation is mainly due to the interaction of the denaturants with the Trp, Tyr and His residues in the protein structure. Therefore, it is clear that ΔG′tr studies is the exploration of the understanding that if a protein consists of more amount of Trp, Tyr or His, it may be denatured or more favorable to denaturation if these AAs individually have a low ΔG′tr values in ILs, as compared to the ΔG′tr for the AAs in water. Since, CT is having more amount of Trp, Tyr and His as compared to the Mb, it may be expected from the above conclusion that the structure of CT by DEAS is less stabilized.32
A comparison between simple GPs (Gly, Gly2, Gly3 and Gly4) in ILs is presented in Fig. 3. In this figure, it can be seen that values are rigorously positive, which indicate that there is unfavorable interaction between ILs and GPs that leads to stabilizing the structure of GPs. Overall, the results in Fig. 3 clearly show that common anions of acetate with alkyl ammonium substituted cations have comparable abilities on stabilizing the GPs. The cation [TEA]+ IL such as TEAA acts as a strong stabilizer, while DEAA is a weak stabilizer for all investigated GPs.41
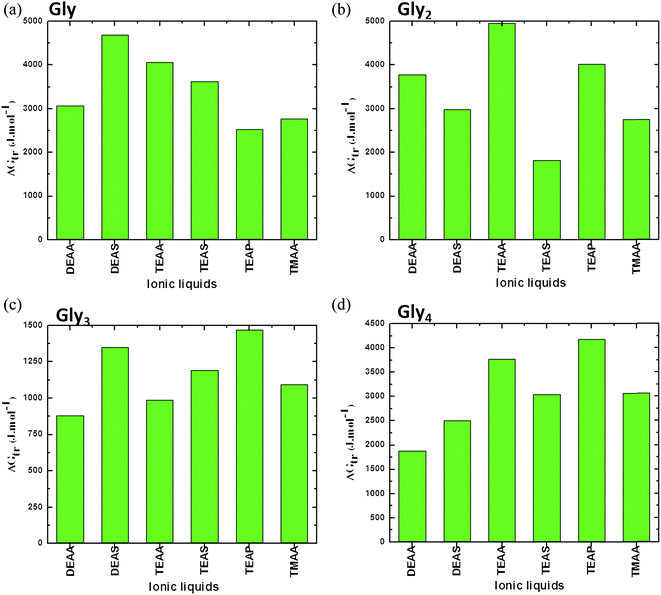 | ||
| Fig. 3 Representation of apparent transfer free energy (ΔG′tr) measurements of glycines (a) Gly, (b) Gly2, (c) Gly3 and (d) Gly4, from water to ammonium family IL (50% (v/v)) solution at 25 °C (data points are collected from ref. 38, 39 and 41). | ||
The stabilizing ability of common anions of sulfate with alkyl ammonium substituted cations follows DEAS > TEAS in all studied GPs, except Gly4, which mainly depends upon the nature of interaction between both alkyl cation and GPs. These results have emphasized that the stability order of the GPs or protein with ILs mainly depends upon the nature of ILs and GPs and the ions of the ILs are extremely sensitive on the structures of biomolecule.41 It can be concluded from these findings that a critical role is played by the anion and alkyl chain length of the cation of the ILs in controlling the protein model compound stability. Clearly, there is no regular order for ammonium-based ILs in stabilizing the GPs; this may be due to variation in the structural arrangements of ILs as well as model compounds.41 Additionally, if we compare each segments in Fig. 3, it is observed that ΔG′tr values for the GPs in [DEA]+ containing ILs are higher for Gly which are reduced when moving towards higher Gly residues (i.e. Gly → Gly4) (except Gly2 in DEAA). This may be due to the reduction in the hydrophobic interactions between the Glyn (n = 2–4) residues and the lower alkyl chain length of the cation [DEA]+ of the ILs, thereby, resulting in the increase ΔG′tr values.
A comparison between the CDs based on the ΔG′tr values in the presence of ILs such as DEAA, TEAA, DEAP, TEAP, DEAS and TEAS is plotted in Fig. 4. The results in Fig. 4 unambiguously reveal that the ΔG′tr values are positive for CDs in ILs, which indicate unfavorable interactions between ILs and CDs. The ΔG′tr values of TEAS with each of c(Ala–Gly), c(Gly–Gly), and c(Ala–Ala) are significantly larger, and there is a large increase with increasing TEAS concentrations as compared to the other ILs.45 On the other hand, the ΔG′tr values are enormously lower for the more hydrophobic character of (Val–Val). CDs tend to stabilize ILs in the following order: TEAS > DEAS > TEAA > DEAA > TEAP > DEAP. Obviously, sulfate anions are strong stabilizers, acetates are moderate stabilizers and phosphate ions are weak stabilizers for CDs.45
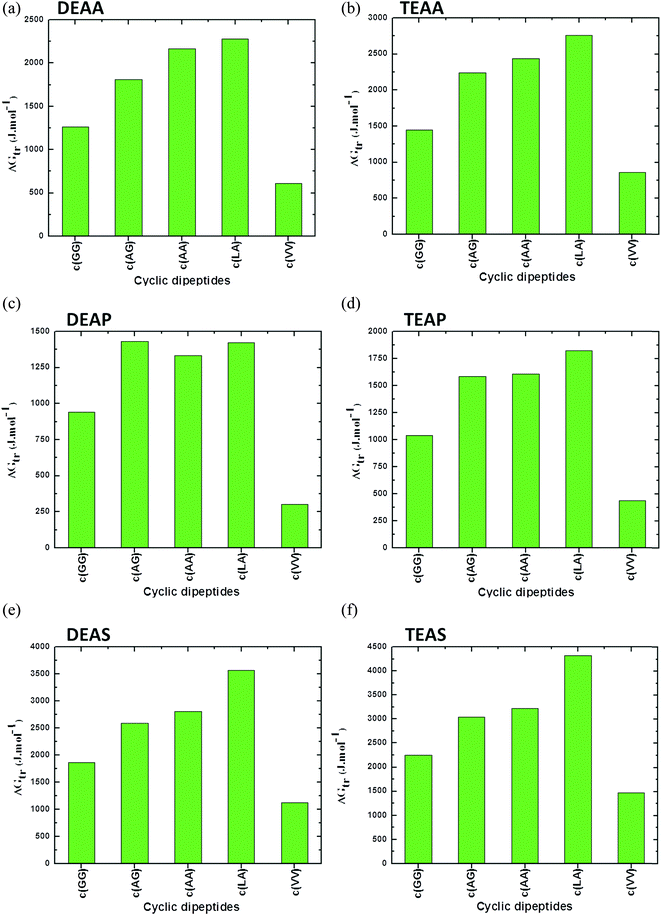 | ||
| Fig. 4 Apparent transfer free energies (ΔG′tr) of cyclic dipeptides from water to ammonium family IL (50% (v/v)) solutions (a) DEAA, (b) TEAA, (c) DEAP, (d) TEAP, (e) DEAS and (f) TEAS at 25 °C (data points are collected from ref. 45). | ||
Significant progress in predicting the thermodynamic properties of GPs in ILs is lacking. However, based on the literature data, it can be observed that the peptide bonds due to combination of similar AAs are comparatively weak in the presence of ILs. As can be seen in Fig. 4, a combination of Leu–Ala (c(Leu–Ala)) results in higher values of ΔG′tr in the presence of DEAA, TEAA, DEAP, TEAP, DEAS and TEAS ILs. This may be assumed to be a strong peptide bond between Leu and Ala as compared to other peptide bonds between similar AAs such as c(GG) or c(VV). Replacing an AA in the protein structure have been reported to stabilize protein against denaturation, however, the contribution of a peptide bond that results due to the interchange of one of the AA on overall protein stability is not reported in literature.
The effects of the ILs on the model compounds can be interpreted essentially in terms of the preferential interactions of the ILs with the aqueous interface and with the AAs surface. As aforementioned, the ILs stabilizing the AAs, GPs and CDs, during this period ILs are excluded from the surfaces of model compounds, where their concentrations near the compounds are lower than those in the bulk solution. IL enhances the water structure and forms the hydration layer with water molecules. During this time, model compound interacts less with hydrated water around the ILs and, therefore, there is negative binding between the ILs and the model compound. Accordingly, unfavourable interactions between biomolecule and ILs are primarily responsible for the model compound stability. During this period, water interacts more favorably with the surface of the model compound. Meanwhile, IL can be excluded from the model compound surface due to the steric repulsion from water molecules. In other words, ILs interact unfavorably with protein surfaces that assist in forming the hydration layer around the protein, further stabilizing it. The schematic illustration of IL exclusion from the model compound surface has been schematically shown in Scheme 4.
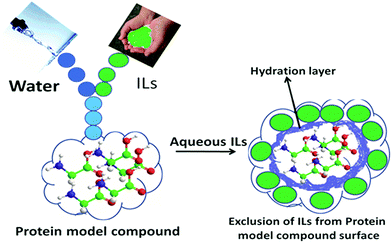 | ||
| Scheme 4 Representation of model compounds in the presence of ILs. IL is excluded from the surface of model compounds due to steric repulsions from the hydration layer (blue colour), obviously unfavorable interactions are occurring between IL and the model compounds surface (ref. 41). Reprinted with permission from (J. Phys. Chem. B, 2012, ,116, 11968). Copyright (2012) American Chemical Society. | ||
As mentioned earlier that the ΔG′tr values are strictly positive for GPs (Gly, Gly2, Gly3 and Gly4 and c(Gly–Gly)) in ammonium-based ILs, which indicate that there is unfavorable interaction between ILs and GPs lead to stabilizing the structure of GPs in these ILs. In contrast, very recently, Varadhi and Venkatesu98 have observed different phenomena for the same GPs in imidazolium-based ILs under the similar experimental conditions. The ILs used in their study have different anions such as chloride (Cl−), bromide (Br−), hydrogen sulfate (HSO4−), acetate (CH3COO−), and thiocyanate (SCN−) and a common 1-butyl-3-methylimidazolium cation, ([Bmim])+.98 It has been shown that the ΔG′tr values of small glycines Gly or Gly2, increase with increasing the concentration of all studied ILs, which indicates the stabilization of the structure of Gly or Gly2 through unfavorable interactions between the Gly or Gly2 and the ions of imidazolium family ILs.
Furthermore, the ΔG′tr values for Gly3 from water to [Bmim][Br] or [Bmim][CH3COO] ILs are monotonically positive while the values are negative for Gly3 in [Bmim][Cl], [Bmim][SCN] or [Bmim][HSO4] of ILs at lower concentrations (≈0.1, ≈ 1.5, or ≈ 1.8 M of these ILs, respectively); later, an increase in ΔG′tr values are observed when, [Bmim][Cl], [Bmim][SCN] or [Bmim][HSO4] of ILs concentration enhance up to 2.5 M. Analogously, [Bmim][Br] increases the positive ΔG′tr values of Gly4 at all studied concentrations whereas the observed ΔG′tr values are negative for Gly4 in [Bmim][CH3COO], [Bmim][Cl], [Bmim][SCN] or [Bmim][HSO4] ILs at lower concentrations (≈0.8, ≈1.4, ≈1.5 or ≈1.75 M ILs, respectively); further, these absolute values are positive. These results indicate a balance between the steric repulsion of IL from the water molecules at the surface of higher glycines and van der Waals attraction between ions of ILs and the peptide bond of the higher glycines.98
The ΔG′tr values of c(Gly–Gly) in aqueous imidazolium-based ILs are distinctly different from that of GPs. The positive ΔG′tr values are found in c(Gly–Gly) in [Bmim][CH3COO], [Bmim][Cl] and [Bmim][SCN] ILs, whereas negative ΔG′tr values were observed for c(Gly–Gly) in the remaining [Bmim][Br] or [Bmim][HSO4] ILs. Surprisingly, the observed negative ΔG′tr values for c(Gly–Gly) in [Bmim][Br] show that Br− IL behaved as a strong destabilizer for the structure of c(Gly–Gly) and this behavior is entirely reversed for this IL behavior in case of glycines, in which [Bmim][Br] behaved as a strong stabilizer for the structures of all glycines. The GPs stability/destability and associations depend on the hydrophobic, hydrophilic and van der Waals interactions between the GPs and co-solvent molecules.98 Apparently, the ΔG′tr values of glycines in ammonium family ILs41 or imidazolium-based ILs98 from Gly to Gly4, decrease with increasing backbone of the glycines. Moreover, it is noticed that the observed ΔG′tr values for GPs in ammonium-based ILs are obviously higher than those of GPs in imidazolium family ILs.41,98
It is noteworthy to compare the ΔG′tr values (Fig. 2) for the Ser in ammonium-based ILs37 (DEAA, DEAS, TEAA, TEAS, TEAP, and TMAA) with those of Ser in imidazolium-based ILs.78 We observed that the ΔG′tr values for Ser in ammonium family ILs are positive as a function of the concentration of these ILs. In contrast, Zafarani-Moattar et al.78 reported that ΔG′tr values for Ser in imidazolium-based ILs (1-(2-carboxyethyl)-3-methylimidazolium chloride) ([HOOCEMIM][Cl]) and 1-carboxymethyl-3-methylimidazolium chloride ([HOOCMMIM][Cl]) are negative and these values decrease with respect to IL concentrations for both systems. The negative values indicate that the interactions between Ser and IL are favorable and both of the imidazolium-based ILs destabilize the Ser structure.78
Taking these aforesaid factors into consideration, we may conclude that the ammonium-based ILs are more biocompatible solvents for the structures of AAs, GPs and CDs than imidazolium-based ILs. This phenomenon is most useful to understand the influence on the variety areas of protein science and biopharmaceutical industry. Moreover, the effects of ILs on structure, activity and stability of biomolecule have to be evaluated and considered for further investigations. The variation in the ΔG′tr of the AA in mixture of water and ILs is directly related to the solubility of the AA in water.97 An increase in the branching of the AA increases its hydrophobicity, the aqueous medium that restricts the motion and the arrangements of the water molecules.99,100 Due to this, low solubility is observed for branched AA in water. In this context, Maheshwari and Dhathathreyan101 have presented the polarity scale based on the tendencies of AA to leave water. Based on their experimental data, the structure of the long chain AA leads to lower solubility in water. Therefore, Ala, Gly and Val are very soluble, His and Leu are moderately, and Trp and Tyr are weakly soluble in water.97 The decrease in the solubility of AAs reflects that the salting-out effect is dominant in aqueous solution. The salting-out of the AA reflects an increase in the stability of the structure of AA in the presence of ILs, whereas, the salting-in effect mainly shows that ILs increase the solubility of the AA in the presence of ILs that indicates the denaturation of AA structures.72 The magnitude of this salting-out/salting-in effect depends on the nature of ILs and generally follows the physical interface of AA structure.
The interpretation of the behavior of AAs in the mixture of water and ILs is difficult to predict. In aqueous medium, there is a balance between competitive interaction of the cation, anion and water molecules with the AA. The combination of these complex interactions determines the preferential interaction as well as solvation of the IL with AA that is helpful in predicting the differences in the solubility limits of the AA in the presence of varying concentrations of the ILs in aqueous solution.80 As proposed by Tome et al.80,81 the salting-in and salting-out phenomena possess a common basis that is the competition between water–AA side chain, IL–AA side chain, and water–IL interactions. There is always a delicate balance between these interactions that is dependent on the relative affinities of the biomolecules to water or to IL (cation or anion). In support to these observations, more recently, Richardt et al.102 analyzed the individual chemical shift differences based on highly resolved one- and two-dimensional NMR spectra and illustrated that NMR spectrum was completely dependent on the interactions between peptides and ILs.
Moreover, the result varies due to the changes in the interactions depending on the nature of the peptide and, most importantly, the composition and the nature of the ILs.69,103 Therefore, it seems that in IL solutions, the electrostatic forces are dominant based on the effect of ions of ILs on their ability to screen effective charges on the AA surface. Additionally, the performance of AA extraction in IL water systems is sensitive to the hydrophobic character and chemical structures of ions in the ILs.97,104
Experimentally, the partitioning behavior of AAs by ILs from aqueous media is mainly influenced by the hydrophobic character of the AAs, indicating the importance of the hydrophobic effect of AAs as a driving force for their partition into a particular IL.90 Wang et al.90 proposed that the electrostatic interactions between the AA surface and ILs are important for the preference of AA towards various ILs. Later, Tome et al.105 proposed that the partitioning behavior of Trp between aqueous and IL phases is ruled by a complex interplay of intermolecular forces between the solute and the IL solvent, such as electrostatic interactions between the charged form of the AA and the ions of the IL. Consequently, the magnitude of the interaction of IL with AA is expected to vary as compared with the solubility of the AA in ILs aqueous solution.106
5.1.The contribution of the peptide backbone unit (or glycyl residue) and various functional groups from water to ILs
The ΔG′tr of a given model protein compound is usually interpreted in terms of group additivity, which is a good approximation method to obtain the contribution of a functional group that is assumed to be independent of neighboring functional groups.41,41,72,74–76 The ΔG′tr represents the change in free energy of each AA upon transferring from water to co-solvent, since ΔG′tr is, by definition, free of solute–solute interactions, which provides information regarding solute–solvent interactions. The values of ΔG′tr are then used to calculate the contribution of peptide backbone unit, i.e., the glycyl residue (–CH2CONH–) from water to co-solvent.41,74,75 and designated by Δg′tr.5.2. Transfer free energy (Δg′tr) contribution of peptide backbone unit of zwitterionic GPs from water to ILs
The transfer free energy contributions of peptide backbone unit of GPs are obtained from the difference between the values ΔG′tr of related to the sequences of Gly to Gly4 as a function of IL concentration, which is schematically shown in Scheme 5. The detailed description of the obtained Δg′tr values is delineated in our earlier articles.41,98 In 2012, Vasantha et al.41 reported the Δg′tr contribution of peptide backbone from water to ammonium-based IL solutions from the ΔG′tr values of GPs in the ILs; the authors observed both positive and negative Δg′tr values, and the values increase with increasing IL concentration. Different values are observed for the Gly residue contribution, depending on the molecule into which the Gly group is inserted. The negative contribution indicates that the interactions between the IL and glycyl residue are favorable. The observed negative values for glycyl residue from water to IL systems suggest that the zwitterionic GPs are significantly denatured by the IL and the destabilization of these Gly peptides increases with increase in the strength of ILs. In other words, the positive contribution indicates that IL is a stabilizer for the glycyl residue of glycine peptides. IL exhibits unfavorable interactions with glycyl residue.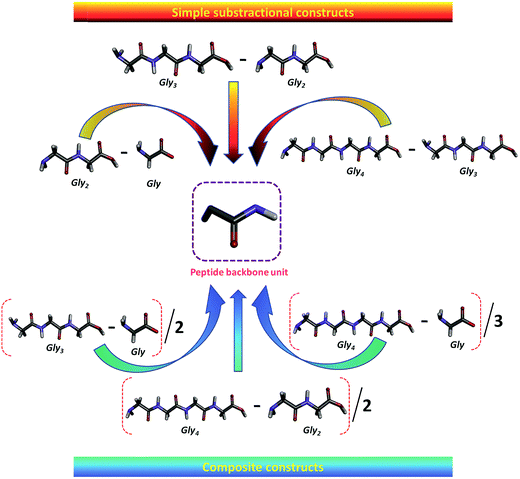 | ||
| Scheme 5 Schematic illustration of the contribution of the peptide backbone unit of glycine peptides (ref. 98). Reprinted with permission from (Ind. Eng. Chem. Res., 2014, 53, 19628). Copyright (2014) American Chemical Society. | ||
Very recently, Govinda and Venkatesu98 reported the contributions (Δg′tr) of the peptide backbone unit of GPs from water to imidazolium-based ILs and observed both positive and negative Δg′tr contribution of peptide backbone unit from water to aqueous imidazolium-based IL solutions. For example, we obtained negative Δg′tr values for [Gly2–Gly] in Br−, Cl− of imidazolium-based ILs solutions, except higher concentrations of Br− and Cl− of ILs, in which we obtained positive values. The negative Δg′tr contribution of peptide backbone unit is observed in the presence of CH3COO−, HSO−4, SCN− ILs (except at higher concentration of SCN−) in the whole concentrations of the ILs, which indicates that the peptide backbone unit is significantly denatured by these ILs. Moreover, the Δg′tr values are negative for [(Gly3–Gly2)], [(Gly4–Gly3)], [(Gly3–Gly1)/2], [(Gly4–Gly2)/2] and [(Gly4–Gly1)/3] in the whole concentration ranges of all ILs (except for [(Gly4–Gly3)], at the lower concentration of Cl−, HSO4− and SCN− ions of ILs). Clearly, the values of Δg′tr for glycine residue are very sensitive to the nature of neighboring groups and dependent on the ionic strength of the solutions.41,98
5.3. Transfer free energy (Δg′tr) contribution of peptide backbone unit of c(Gly–Gly) from water to ILs
c(Gly–Gly), was chosen as a model compound of typical interactions found in proteins because it is the cyclic dimer of glycine. The hydrogen-bonded structure makes c(Gly–Gly), a good model compound for hydrogen bonding that takes place within the peptide backbone of a protein.4,41,45,72 Obviously, c(Gly–Gly), is a good peptide model for the peptide backbone unit because it contains two peptide groups in the structure that expose the peptide unit to the solvent. Consequently, the ΔG′tr value of c(Gly–Gly) from water to IL solutions is divided by two to obtain the contribution of one peptide backbone unit as shown in Scheme 6.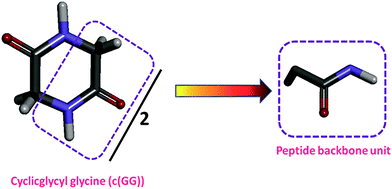 | ||
| Scheme 6 Schematic illustration of determining the contribution of the peptide backbone unit of c(Gly–Gly) (ref. 98). Reprinted with permission from (Ind. Eng. Chem. Res., 2014, 53, 19628). Copyright (2014) American Chemical Society. | ||
It has been shown that the Δg′tr values for the peptide backbone unit of c(Gly–Gly) from water to ammonium-based IL solutions are positive and are observed to increase with increasing IL concentration in aqueous solution.41 These findings provide an unfavorable transfer free energy of the peptide backbone unit of c(Gly–Gly) from water to ammonium-based ILs. On the other hand, the Δg′tr values are positive for peptide backbone unit of c(Gly–Gly) from water to CH3COO−, Cl−, SCN− ions of IL solutions (except at lower concentration of SCN−) and this contribution increases with increasing IL concentrations. Furthermore, the Δg′tr values are negative for peptide backbone unit of c(Gly–Gly) from water to HSO4− and Br− ions of these ILs in the solution, which indicate a favorable interaction between this residue with ions of ILs. The obtained Δg′tr behavior is quite contrasting to the ΔG′tr of GPs in imidazolium-based ILs.98 These results show that the value Δg′tr of for the peptide backbone unit is sensitive to the nature of the neighboring groups and is dependent on the ILs. Note that some of the protecting ILs interact favorably with some of the glycyl residue. This behavior is quite different from the model compound contributions, which exhibit unfavorable interactions with protecting ILs.41,98
5.4. Transfer free energy (Δg′tr) contribution of the peptide bond (amide unit), apolar hydrogens and functional groups from water to IL solutions
Attri and Venkatesu45 observed that the Δg′tr values for one peptide bond as well as one apolar hydrogen is positive, therefore, the Δg′tr contribution is unfavorable for peptide bonds or for apolar hydrogen groups with ammonium-based ILs. Furthermore, the same authors also observed that the Δg′tr values of both alanyl or valyl residue contribution from water to ammonium-based ILs are positive. The magnitude and sign of these values have no significant difference from those of the peptide backbone unit of c(Gly–Gly). It is clear that ILs interact unfavorably with the alanyl or valyl residue, similar to the interactions between the glycine residues and ammonium-based ILs.45 The Δg′tr values of the alanyl side chain and leucyl side chain contribution were calculated and found to be positive from water to ammonium-based ILs, except at higher concentration of DEAP by the same research group.Alanyl and leucyl side chains contribute favorable interaction with DEAP for few cases only and unfavorable interaction with other ILs. These types of interactions are described as providing an increase in structural order of the solvent systems, the structural arrangements, and the positions of the side chains in the structure of the CDs. The results of the Δg′tr values of the side chains show marginal free energy changes at lower concentrations of some ILs while it becomes more at higher concentrations of ILs. One of the important conclusions drawn from these results is that some of the protecting ILs interacts favorably with some of the side chains. This behavior is quite different from the peptide bond or peptide backbone unit contributions, which exhibit unfavourable interactions with protecting ILs. Note that the peptide bond or the peptide backbone unit (containing amide) is the most numerous group, responsible for protein stability. However, side chains play a less significant role in protein stability.45
6. Conclusion
The literature surveys of the thermodynamic analysis are informative which brings forward the influences of solvation behavior of ammonium and imidazolium-based ILs on AAs. However, the interest is drawn more towards the ammonium-based ILs. It reveals that the ILs such as DEAA, DEAS, TEAA, TEAS, TEAP and TMAA have high biocompatibility and can stabilize AAs structures through unfavorable interactions. Majority of the results show the decrease in the solubility (salting-out) of the protein model compounds with increasing ammonium-based ILs concentration indicate towards the unfavorable interaction between ions of ILs and the surface of protein model compounds. In such cases the obtained ΔG′tr values are positive, which reveals that the structure of protein model compounds are stabilized by these ILs. The magnitude of salting-out or salting-in effect depends on the nature of ions of ILs and physical interface and variable surfaces of the model compounds. Most of the ammonium-based ILs are acted as biocompatible solvents for the model compounds whereas the imidazolium-based ILs behave as non biocompatible solvents for the structures of protein model compounds. Hence, the combined conclusions of the obtained results converge to the point that the ammonium based ILs are very efficient co-solvent media which are capable of stabilizing the structure of proteins. Moreover, we believe that the collection of these experimental findings would help to provide information to improve the ammonium-based ILs as an attractive and alternative reaction media for biomolecules and would be the most preferred ILs among the biophysical chemists in the future. We believe that these thermodynamic findings will encourage the study of model protein compounds in IL medium in future as well.Conflict of interest
The authors declare no competing financial interest.Abbreviations
| Ionic Liquids | ILs |
| Ionic Liquid | IL |
| Amino acids | AAs |
| Amino acid | AA |
| Glycine | Gly |
| Alanine | Ala |
| Arginine | Arg |
| Valine | Val |
| Leucine | Leu |
| Tryptophan | Trp |
| Tyrosine | Tyr |
| Phenylalanine | Phe |
| Glutamic acid | Glu |
| Lysine | Lys |
| Histidine | His |
| Theronine | Thr |
| Glutamine | Gln |
| Serine | Ser |
| Glycine peptides | GPs |
| Diglycine | Gly2 |
| Triglycine | Gly3 |
| Tetraglycine | Gly4 |
| Cyclic dipeptides | CDs |
| Cyclic glycyl–glycine | c(Gly–Gly) |
| Cyclic alanine–glycine | c(Ala–Gly) |
| Cyclic alanine–alanine | c(Ala–Ala) |
| Cyclic leucine–alanine | c(Leu–Ala) |
| Cyclic valine–valine | c(Val–Val) |
| α-Chymotrypsin | CT |
| Myoglobin | Mb |
| Bovine serum albumin | BSA |
| 1-Hexyl-3-methylimidazolium tetrafluoroboarte | [Hexmim][BF4] |
| 1-Decyl-3-methylimidazolium tetrafluoroborate | [Dmim][BF4] |
| 1-Butyl-3-methylimidazolium hexafluorophosphate | [Bmim][PF6] |
| 1-Butyl-3-methylimidazolium bromide | [Bmim][Br] |
| 1-Butyl-3-methylimidazolium chloride | [Bmim][Cl] |
| 1-Butyl-3-methylimidazolium thiocyanate | [Bmim][SCN] |
| 1-Butyl-3methylimidazolium hydrogensulfate | [Bmim][HSO4] |
| 1-Butyl-3-methylimidazolium acetate | [Bmim][CH3COO] |
| 1-Hexyl-3-methylimidazolium hexafluorophosphate | [Hexmim][PF6] |
| 1-Octyl-3-methylimidazolium tetrafluoroborate | [Omim][BF4] |
| Diethyl ammonium acetate ([Et2NH][CH3COO]) | DEAA |
| Diethyl ammonium sulfate ([Et2NH][HSO4]) | DEAS |
| Triethyl ammonium acetate ([Et3NH][CH3COO]) | TEAA |
| Triethyl ammonium sulfate ([Et3NH][HSO4]) | TEAS |
| Triethyl ammonium phosphate ([Et3NH][H2PO4]) | TEAP |
| Trimethyl ammonium acetate ([Me3NH][CH3COO]) | TMAA |
| (1-(2-Carboxyethyl)-3-methylimidazolium chloride) | [HOOCEMIM][Cl] |
| 1-Carboxymethyl-3-methylimidazolium chloride | [HOOCMMIM][Cl] |
| Apparent transfer free energy of protein model compound | ΔG′tr |
| Apparent transfer free energy of peptide backbone unit or functional groups | Δg′tr |
| Aqueous biphasic systems | ABS |
Acknowledgements
We acknowledge the financial support from the Department of Science and Technology (DST, Grant No. SB/SI/PC-109/2012), Council of Scientific Industrial Research (CSIR, Grant No. 01 (2713) 13/EMR-II), Department of Biotechnology (DBT, Grant No. BT/PR5287/BRB/10/1068/2012), New Delhi, India, University of Delhi (R & D Grant No. RC/2015/9677) and DU-DST Purse Grant (Grant No. CD/2015/1534).References
- T. E. Creighton and W. H. Freeman, Save theory wrang, New York, 1992 Search PubMed.
- S. Doonan, Peptides and proteins, Royal Society of Chemistry, Cambridge, UK, 2002 Search PubMed.
- P. Venkatesu, M. J. Lee and H. M. Lin, J. Phys. Chem. B, 2007, 111, 9045–9056 CrossRef CAS PubMed.
- P. Venkatesu, M. J. Lee and H. M. Lin, Arch. Biochem. Biophys., 2007, 466, 106–115 CrossRef CAS PubMed.
- P. Venkatesu, M. J. Lee and H. M. Lin, Biochem. Eng. J., 2006, 32, 157–170 CrossRef CAS.
- A. Pertsemlidis, A. M. Saxena, A. K. Soper, T. Head-Gordon and R. M. Glaeser, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 10769–10774 CrossRef CAS.
- S. M. Malathy Sony, K. Saraboji, N. Sukumar and M. N. Ponnuswamy, Biophys. Chem., 2006, 120, 24–31 CrossRef CAS PubMed.
- L. Xu, X. Wang, R. Lin and D. Sun, J. Chem. Thermodyn., 2005, 37, 371–375 CrossRef CAS.
- Y. Liu and D. W. Bolen, Biochemistry, 1995, 34, 12884–12891 CrossRef CAS PubMed.
- A. W. Hakin, H. Høiland and G. R. Hedwig, Phys. Chem. Chem. Phys., 2000, 2, 4850–4857 RSC.
- M. Auton and D. W. Bolen, Biochemistry, 2004, 43, 1329–1342 CrossRef CAS PubMed.
- P. Attri and P. Venkatesu, Thermochim. Acta, 2011, 526, 143–150 CrossRef CAS.
- S. Ortiz-Costa, M. M. Sorenson and M. Sola-Penna, FEBS J., 2008, 275, 3388–3396 CrossRef CAS PubMed.
- W. Kauzmann, Adv. Protein Chem., 1959, 14, 1–63 CrossRef CAS PubMed.
- C. Tanford, J. Am. Chem. Soc., 1962, 84, 4240–4247 CrossRef CAS.
- A. Kumar and P. Venkatesu, Chem. Rev., 2012, 112, 4283–4307 CrossRef CAS PubMed.
- K. Fujita, D. R. Macfarlane and M. Forsyth, Chem. Commun., 2005, 4804–4806 RSC.
- A. Kumar, A. Rani, P. Venkatesu and A. Kumar, Phys. Chem. Chem. Phys., 2014, 16, 15806–15810 RSC.
- L. G. Tamar and J. D. Calum, Chem. Rev., 2015, 115, 11379–11448 CrossRef PubMed.
- R. Hayes, G. G. Warr and R. Atkin, Chem. Rev., 2015, 115, 6357–6426 CrossRef CAS PubMed.
- J. Li, Y. Lu, D. Yang, Q. Sun, Y. Liu and H. Zhao, Biomacromolecules, 2011, 12, 1860–1867 CrossRef CAS PubMed.
- T. Sato, G. Masuda and K. Takagi, Electrochim. Acta, 2004, 49, 3603–3611 CrossRef CAS.
- I. Jha and P. Venkatesu, ACS Sustainable Chem. Eng., 2016, 4, 413–421 CrossRef CAS.
- M. Deetlefs and K. R. Seddon, Chim. Oggi-Chem. Today, 2006, 24, 16–23 CAS.
- P. Tundo, A. Perosa and F. Zecchini, Ionic liquids: ‘Designer’ solvents for green chemistry, in Methods and Reagents for Green Chemistry: An Introduction, Wiley, New York, 2007, pp. 105–130 Search PubMed.
- M. Poliakoff, J. M. Fitzpatrick, T. R. Farren and P. T. Anastas, Science, 2002, 297, 807–810 CrossRef CAS PubMed.
- A. Kumar, A. Rani and P. Venkatesu, New J. Chem., 2015, 39, 938–952 RSC.
- A. Kumar and P. Venkatesu, RSC Adv., 2013, 3, 362–367 RSC.
- P. Attri, P. Venkatesu and A. Kumar, Org. Biomol. Chem., 2012, 10, 7475–7478 CAS.
- P. Attri, P. Venkatesu, A. Kumar and N. Byrne, Phys. Chem. Chem. Phys., 2011, 13, 17023–17026 RSC.
- P. Attri, P. Venkatesu and A. Kumar, Phys. Chem. Chem. Phys., 2011, 13, 2788–2796 RSC.
- P. Attri and P. Venkatesu, Process Biochem., 2013, 48, 462–470 CrossRef CAS.
- A. Kumar and P. Venkatesu, Process Biochem., 2014, 49, 2158–2169 CrossRef CAS.
- I. Jha, P. Attri and P. Venkatesu, Phys. Chem. Chem. Phys., 2014, 16, 5514–5526 RSC.
- A. Kumar and P. Venkatesu, RSC Adv., 2014, 4, 4487–4499 RSC.
- T. Vasantha, A. Kumar, P. Attri, P. Venkatesu and R. S. Ramadevi, Protein Pept. Lett., 2014, 21, 15–24 CrossRef CAS PubMed.
- T. Vasantha, T. Kavitha, A. Kumar, P. Venkatesu and R. S. Ramadevi, J. Mol. Liq., 2015, 208, 130–136 CrossRef CAS.
- T. Vasantha, P. Attri, P. Venkatesu and R. S. Ramadevi, J. Chem. Thermodyn., 2013, 56, 21–31 CrossRef CAS.
- T. Vasantha, P. Attri, P. Venkatesu and R. S. Ramadevi, J. Chem. Thermodyn., 2012, 45, 122–136 CrossRef CAS.
- T. Vasantha, A. Kumar, P. Attri, P. Venkatesu and R. S. Ramadevi, Fluid Phase Equilib., 2012, 335, 39–45 CrossRef CAS.
- T. Vasantha, P. Attri, P. Venkatesu and R. S. Ramadevi, J. Phys. Chem. B, 2012, 116, 11968–11978 CrossRef CAS PubMed.
- P. Attri, I. Jha, E. H. Choi and P. Venkatesu, Int. J. Biol. Macromol., 2014, 69, 114–123 CrossRef CAS PubMed.
- P. Attri and P. Venkatesu, Int. J. Biol. Macromol., 2012, 51, 119–128 CrossRef CAS PubMed.
- P. Attri and P. Venkatesu, J. Chem. Thermodyn., 2012, 52, 78–88 CrossRef CAS.
- P. Attri and P. Venkatesu, Phys. Chem. Chem. Phys., 2011, 13, 6566–6575 RSC.
- I. Jha, A. Kumar and P. Venkatesu, J. Phys. Chem. B, 2015, 119, 8357–8368 CrossRef CAS PubMed.
- T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef CAS PubMed.
- I. Jha and P. Venkatesu, Phys. Chem. Chem. Phys., 2015, 17, 20466–20484 RSC.
- T. Kavitha, P. Attri, P. Venkatesu and R. S. Ramadevi, J. Phys. Chem. B, 2012, 116, 4561–4574 CrossRef CAS PubMed.
- R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792–793 CrossRef PubMed.
- K. R. Seddon, J. Chem. Technol. Biotechnol., 1997, 68, 351–356 CrossRef CAS.
- P. M. Reddy and P. Venkatesu, J. Phys. Chem. B, 2011, 115, 4752–4757 CrossRef CAS PubMed.
- J. S. Wilkes, Green Chem., 2002, 4, 73–80 RSC.
- P. M. Reddy, R. Umapathi and P. Venkatesu, Phys. Chem. Chem. Phys., 2014, 16, 10708–10718 RSC.
- T. Welton, Green Chem., 2011, 13, 225 RSC.
- K. S. Egorova and V. P. Ananikov, ChemSusChem, 2014, 7, 336–360 CrossRef CAS PubMed.
- M. Petkovic, K. R. Seddon, L. P. N. Rebelo and C. S. Pereira, Chem. Soc. Rev., 2011, 40, 1383–1403 RSC.
- M. Moniruzzaman, K. Nakashima, N. Kamiya and M. Goto, Biochem. Eng. J., 2010, 48, 295–314 CrossRef CAS.
- F. van Rantwijk and R. A. Sheldon, Chem. Rev., 2007, 107, 2757–2785 CrossRef CAS PubMed.
- C. Branden and J. Tooze, Principles that Govern the Folding of Protein Chain, Garland Punlishing, Ink., New York, 2nd edn, 1999 Search PubMed.
- D. Tietze, H. Breitzke, D. Imhof, E. Kothe, J. Weston and G. Buntkowsky, Chem.–Eur. J., 2009, 15, 517–523 CrossRef CAS PubMed.
- C. B. Anfinsen, Science, 1973, 181, 223–230 CAS.
- R. D. Schaeffer, A. Fersht and V. Daggett, Curr. Opin. Struct. Biol., 2008, 18, 4–9 CrossRef CAS PubMed.
- S. Chandrudu, P. Simerska and I. Toth, Molecules, 2013, 18, 4373–4688 CrossRef CAS PubMed.
- S. Ortiz-Costa, M. M. Sorenson and M. Sola-Penna, Arch. Biochem. Biophys., 2002, 408, 272–278 CrossRef CAS PubMed.
- F. Guzmán, S. Barberis and A. Illanes, Electron. J. Biotechnol., 2007, 10, 279–314 Search PubMed.
- J. C. Plaquevent, J. Levillain, F. Guillen, C. Malhiac and A. C. Gaumont, Chem. Rev., 2008, 108, 5035–5060 CrossRef CAS PubMed.
- A. A. Tietze, P. Heimer, A. Stark and D. Imhof, Molecules, 2012, 17, 4158–4185 CrossRef CAS PubMed.
- M. M. Seitkalieva, V. V. Kachala, K. S. Egorova and V. P. Ananikov, ACS Sustainable Chem. Eng., 2015, 3, 357–364 CrossRef CAS.
- K. Ueno, H. Tokuda and M. Watanabe, Phys. Chem. Chem. Phys., 2010, 12, 1649–1658 RSC.
- P. H. Yancey, M. E. Clark, S. C. Hand, R. D. Bowlus and G. N. Somero, Science, 1982, 217, 1214–1222 CAS.
- P. Venkatesu, M. J. Lee and H. M. Lin, Biochem. Eng. J., 2008, 38, 326–340 CrossRef CAS.
- P. Venkatesu, M. J. Lee and H. M. Lin, J. Phys. Chem. B, 2009, 113, 5327–5338 CrossRef CAS PubMed.
- Y. Nozaki and C. Tanford, J. Biol. Chem., 1963, 238, 4074–4081 CAS.
- Y. Nozaki and C. Tanford, J. Biol. Chem., 1965, 240, 3568–3573 CAS.
- Y. Nozaki and C. Tanford, J. Biol. Chem., 1970, 245, 1648–1652 CAS.
- D. S. Tomar, D. Asthagiri and V. Weber, Biophys. J., 2013, 105, 1482–1490 CrossRef CAS PubMed.
- M. T. Zafarani-Moattar, B. Asadzadeh, A. Shahrisa and M. G. Nazari, J. Chem. Thermodyn., 2015, 80, 49–58 CrossRef CAS.
- C. L. S. Louros, A. F. M. Cláudio, M. S. S. Neves Catarina, M. G. Freire, I. M. Marrucho, J. Pauly and J. A. P. Coutinho, Int. J. Mol. Sci., 2010, 11, 1777–1791 CrossRef CAS PubMed.
- L. I. N. Tomé, M. Jorge, J. R. B. Gomes and J. A. P. Coutinho, J. Phys. Chem. B, 2012, 116, 1831–1842 CrossRef PubMed.
- L. I. N. Tomé, M. D. Perez, A. F. M. Claudio, M. G. Freire, I. M. Marrucho, O. Cabeza and J. A. P. Coutinho, J. Phys. Chem. B, 2009, 113, 13971–13979 CrossRef PubMed.
- A. Seduraman, P. Wu and M. Klähn, J. Phys. Chem. B, 2012, 116, 296–304 CrossRef CAS PubMed.
- Y. Kohno and H. Ohno, Chem. Commun., 2012, 48, 7119–7130 RSC.
- S. Fang and D. H. Ren, J. Chem. Eng. Data, 2013, 58, 845–850 CrossRef CAS.
- H. Shekaari and F. Jebali, J. Solution Chem., 2010, 39, 1409–1427 CrossRef CAS.
- A. A. Tietze, F. Bordusa, R. Giernoth, D. Imhof, T. Lenzer, A. Maa, C. M. Klaus, I. Neundorf, K. Oum, D. Reith and A. Stark, ChemPhysChem, 2013, 14, 4044–4064 CrossRef CAS PubMed.
- M. D. Pérez, L. I. N. Tomé, M. G. Freire, I. M. Marruchob, O. Cabezaa and J. A. P. Coutinho, Sep. Purif. Technol., 2010, 72, 85–91 CrossRef.
- M. G. Freire, A. F. Cláudio, J. M. Araújo, J. A. P. Coutinho, I. M. Marrucho, J. N. C. Lopes and L. P. Rebelo, Chem. Soc. Rev., 2012, 41, 4966–4995 RSC.
- O. Rodriguez, P. P. Madeira and E. A. Macedo, Proceedings of European Congress of Chemical Engineering (ECCE-6), Copenhagen, 16-20 September, 2007 Search PubMed.
- J. Wang, Y. Pei, Y. Zhao and Z. Hu, Green Chem., 2005, 7, 196–202 RSC.
- J. T. Vivian and P. R. Callis, Biophys. J., 2001, 80, 2093–2109 CrossRef CAS PubMed.
- M. T. Z. Moattar and S. Hamzehzadeh, Biotechnol. Prog., 2011, 27, 986–997 CrossRef PubMed.
- G. Absalan, M. Akhond and L. Sheikhian, Amino Acids, 2010, 39, 167–174 CrossRef CAS PubMed.
- L. Cammarata, S. G. Kazarian, P. A. Salter and T. Welton, Phys. Chem. Chem. Phys., 2001, 3, 5192–5200 RSC.
- H. Tsunekawa, A. Narumi, M. Sano, A. Hiwara, M. Fujita and H. Yokoyama, J. Phys. Chem. B, 2003, 107, 10962–10966 CrossRef CAS.
- S. Dreyer and U. Kragl, Biotechnol. Bioeng., 2008, 99, 1416–1424 CrossRef CAS PubMed.
- A. Kumar, P. Venkatesu, M. Taha and M. J. Lee, Curr. Biochem. Eng., 2014, 1, 125–140 CrossRef CAS.
- V. Govinda and P. Venkatesu, Ind. Eng. Chem. Res., 2014, 53, 19628–19642 CAS.
- Y. Pei, Z. Li and J. Wang, J. Chromatogr., 2012, 1231, 2–7 CrossRef CAS PubMed.
- Y. C. Pei, J. J. Wang, X. P. Xuan and X. J. Lu, Sep. Purif. Technol., 2009, 64, 288–295 CrossRef CAS.
- R. Maheshwari and A. Dhathathreyan, J. Colloid Interface Sci., 2004, 277, 79–83 CrossRef CAS PubMed.
- A. Richardt, C. Mrestani-Klaus and F. Bordusa, J. Mol. Liq., 2014, 192, 9–18 CrossRef CAS.
- J. L. Huang, M. E. Noss, K. M. Schmidt, L. Murray and M. R. Bunagan, Chem. Commun., 2011, 47, 8007–8009 RSC.
- D. Constantinescu, H. Weingärtner and C. Herrmann, Angew. Chem., Int. Ed., 2007, 46, 8887–8889 CrossRef CAS PubMed.
- L. I. N. Tome, V. R. Catambas, A. R. R. Teles, M. G. Freire, I. M. Marrucho and J. A. P. Coutinho, Sep. Purif. Technol., 2010, 72, 167–173 CrossRef CAS.
- Y. Shim, H. J. Kim and Y. J. Jung, Bull. Korean Chem. Soc., 2012, 33, 3601–3606 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |