Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development of a D–π–A pyrazinium photosensitizer possessing singlet oxygen generation†
Yousuke
Ooyama
*,
Toshiaki
Enoki
and
Joji
Ohshita
*
Department of Applied Chemistry, Graduate School of Engineering, Hiroshima University, Higashi-Hiroshima 739-8527, Japan. E-mail: yooyama@hiroshima-u.ac.jp; Fax: +81-82-424-5494
First published on 8th January 2016
Abstract
(D–π–)2A pyrazinium dyes (OEJ-1 and OEJ-2) bearing a counter anion (X− = Br− or I−) have been newly developed as a photosensitizer possessing singlet oxygen (1O2) generation. The two dyes show specific solvatochromism, leading to a large bathochromic shift of the photoabsorption band in halogenated solvents, compared to polar and non-polar solvents. The effects of the counter anion and solvents on the 1O2 generation efficiency such as ΦΔ and the rate constant (Kobs) have been investigated. It was revealed that OEJ-2 (X− = I−) exhibits a higher 1O2 quantum yield (ΦΔ) than OEJ-1 (X− = Br−). This result indicates that the (D–π–)2A pyrazinium dyes possess the ability to generate 1O2 under visible light irradiation, due to the effective intersystem crossing (ISC) from the singlet excited state of the photosensitizer (1S*) to the triplet excited state (3S*) by the superior heavy-atom effect of I− ion as the counter anion. Moreover, it was found that THF and dichloromethane are favorable solvents for the (D–π–)2A pyrazinium dyes to efficiently generate 1O2 compared with the polar solvents such as acetonitrile and DMSO. On the basis of the 1O2 quantum yield, the rate constant for 1O2 generation, the HOMO and LUMO energy levels of OEJ-1 and OEJ-2, and density functional theory (DFT) calculation, the photoabsorption and 1O2 generation properties of the D–π–A pyrazinium dyes are discussed.
Introduction
Photosensitizers possessing the ability to generate singlet oxygen (1O2) have received considerable attention in recent years from the viewpoint of not only fundamental study in photochemistry and photophysics, but also their potential applications in photodynamic therapy (PDT).1–41O2 generally occurs through the following processes: initially the photosensitizer absorbs light (hν) to generate the singlet excited state of the photosensitizer (1S*), then the photoexcited dye (1S*) undergoes intersystem crossing (ISC) to generate the triplet excited state (3S*). Subsequent energy transfer from the photoexcited dye (3S*) to triplet oxygen (3O2) produces 1O2. Thus, to enhance ISC efficiency is one of the most effective strategies to generate high 1O2 quantum yield. For this purpose, many kinds of photosensitizers exhibiting high 1O2 generation efficiency for PDT have been developed, including organic dyes such as methylene blue5 and rose bengal,6 porphyrin dyes,7,8 phthalocyanines,9 boron dipyrromethene (BODIPY) dyes,10–12 fullerene derivatives13,14 and ruthenium (Ru)15 and iridium (Ir) complexes,16 and the mechanisms of 1O2 generation by the photosensitizers were investigated.1–4,17 However, there have been few efforts to develop new organic photosensitizers possessing the ability to generate 1O2.18Thus, in this work, to gain insight into a direction in molecular design toward creating new photosensitizer family possessing 1O2 generation, we have developed (D–π–)2A pyrazinium dyes (OEJ-1 and OEJ-2) bearing bromide ion (Br−) or iodide ion (I−) as a counter anion (Scheme 1). The heavy atoms such as bromine and iodine would be expected to facilitate ISC by the heavy-atom effect. Moreover, D–π–A pyrazinium dyes have an advantage over the conventional photosensitizers in carrying out the fundamental study on 1O2 generation, that is, we can obtain a great deal of useful knowledge for the relationship between the molecular structures and 1O2 generation efficiency, by easily exchanging the counter ion. Interestingly, it was found that the (D–π–)2A pyrazinium dyes show specific solvatochromism, leading to a large bathochromic shift of absorption band in halogenated solvents, compared to polar and non-polar solvents. Therefore, the effects of the counter anion and solvents on the 1O2 generation efficiency have been investigated. On the basis of 1O2 quantum yield (ΦΔ), rate constant (Kobs) for 1O2 generation, the HOMO and LUMO energy levels of OEJ-1 and OEJ-2, and density functional theory (DFT) calculation, the photoabsorption and 1O2 generation properties of the (D–π–)2A pyrazinium dyes are discussed.
Results and discussion
Synthesis
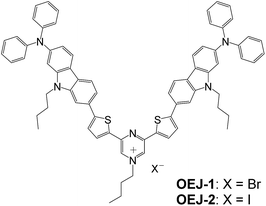
The (D–π–)2A pyrazinium dyes (OEJ-1 and OEJ-2) were synthesized from the (D–π–)2A fluorescent dye OUK-2 (ref. 19) and the corresponding n-butyl halide (Scheme 2).Photoabsorption properties
The photoabsorption spectra of OEJ-1 and OEJ-2 in various solvents (THF, acetonitrile, DMSO and dichloromethane) are shown in Fig. 1 and their optical data are summarized in Table 1. The two dyes show a broad absorption band (λabs) at around 500–700 nm, which is assigned to the intramolecular charge-transfer (ICT) excitation from electron donor moiety (diphenylamino group) to electron acceptor moiety (pyrazinium group). In all the four solvents, the λabs for ICT band of OEJ-2 occurs at a longer wavelength than of OEJ-1. Interestingly, the two dyes showed the specific solvatochromism as with the previously reported D–π–A pyridinium dyes,20 leading to a large bathochromic shift of absorption band in halogenated solvent such as dichloromethane, compared with that in polar and non-polar solvents; the λabs for ICT bands of OEJ-1 and OEJ-2 in dichloromethane occurs at a longer wavelength by ca. 30 nm and ca. 70 nm, respectively, than those in acetonitrile. It is worthy to note here that the specific solvatochromism depends on the counter anion of the (D–π–)2A pyrazinium dyes, that is, the bathochromic shifts of ICT band for OEJ-2 bearing I− ion is larger than that of OEJ-1 bearing Br− ion. | ||
| Fig. 1 Photoabsorption spectra of (a) OEJ-1 and (b) OEJ-2 in THF, acetonitrile, DMSO and dichloromethane (CH2Cl2). | ||
| Dye | Solvent | λ abs/nm for ICT band | ε/M−1 cm−1@λabs = 509 nm | Φ Δ a | K obs b/min−1 |
|---|---|---|---|---|---|
| a 1O2 quantum yield (relative decomposition rate of DPBF), with Rose Bengal (RB) as standard (ΦΔ = 0.80 in methanol,15 see Fig. S4) and 1,3-diphenylisobenzofuran (DPBF) as 1O2 scavenger. These values were estimated under an assumption that the reactivity of singlet oxygen is independent of the kind of solvents. b First-order rate constant for the reaction of DPBF with 1O2 generated upon photoexcitation of OEJ-1 or OEJ-2. The Kobs for RB is 0.250 min−1 (see Fig. S5). c Too low. d Estimated from the slope for the range of 5–10 min in Fig. 9b. | |||||
| OEJ-1 | THF | 490 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.19 | 0.034 |
| Acetonitrile | 490 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
—c | —c | |
| DMSO | 490 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
—c | 0.004 | |
| Dichloromethane | 520 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
—c | 0.006 | |
| OEJ-2 | THF | 500 | 3600 | 0.22 | 0.016 |
| Acetonitrile | 500 | 7600 | 0.05 | 0.032 | |
| DMSO | 500 | 8500 | 0.03 | 0.006 | |
| Dichloromethane | 570 | 9700 | 0.07 | 0.160d | |
Electrochemical properties
The electrochemical properties of OEJ-1 and OEJ-2 were determined by cyclic voltammetry (CV) in acetonitrile containing 0.1 M tetrabutylammonium perchlorate (Bu4NClO4). The potentials were referred to ferrocene/ferrocenium (Fc/Fc+) as the internal reference (Fig. 2). For OEJ-2, the oxidation wave for the iodide counter ion was observed at around 0.05 V. The oxidation waves were observed at 0.43 V for OEJ-1 and 0.35 V for OEJ-2, respectively, vs. Fc/Fc+ (Table 1). The corresponding reduction waves appeared at 0.36 V for OEJ-1 and 0.18 V for OEJ-2, respectively. However, the oxidation and corresponding reduction waves are reversible for OEJ-1, but irreversible for OEJ-2. In fact, at second cycle OEJ-1 also showed the reversible oxidation wave, but OEJ-2 showed cathodic shift by ca. 0.1 V for the oxidation wave as well as disappearance of the oxidation wave for the iodide counter ion (Fig. S3†). The HOMO energy level vs. vacuum level is −5.20 eV for OEJ-1 and −5.07 eV for OEJ-2, respectively, which was evaluated through equation −[Eox1/2 + 4.8]eV from the half-wave potential for oxidation (Eox1/2 = 0.40 V vs. Fc/Fc+ for OEJ-1 and 0.27 V vs. Fc/Fc+ for OEJ-2). On the other hand, the LUMO energy level is −3.20 eV for OEJ-1 and −3.07 eV for OEJ-2, respectively, which was estimated from the HOMO and the onset of photoabsorption spectra (620 nm; 2.0 eV for both OEJ-1 and OEJ-2) in acetonitrile. | ||
| Fig. 2 Cyclic voltammograms of (a) OEJ-1 and (b) OEJ-2 in acetonitrile containing 0.1 M Bu4NClO4 at the first cycle. The arrow denotes the direction of the potential scan. | ||
Theoretical calculations
In order to examine the HOMO and LUMO of OEJ-1 and OEJ-2, the molecular orbitals of the (D–π–)2A pyrazinium dye cation (OEJ) was calculated using density functional theory (DFT) at the B3LYP/6-31G(d,p) level (Fig. 3).21 The DFT calculation for the dye cation indicates that the HOMO is mostly localized on the diphenylamine–carbazole moiety containing a thiophene ring. On the other hand, the LUMO is mainly concentrated on pyrazinium moiety. Accordingly, the DFT calculations reveal that excitation of the dye upon light irradiation induces a strong ICT from the diphenylamine–carbazole moiety to the pyrazinium moiety. | ||
| Fig. 3 (a) HOMO and (b) LUMO of OEJ cation by the density functional theory (DFT) calculations at B3LYP/6-31G(d,p) level. | ||
1O2 generation by (D–π–)2A pyrazinium dye
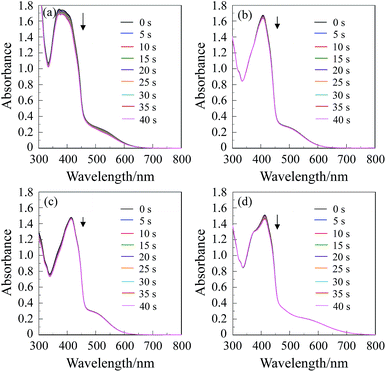
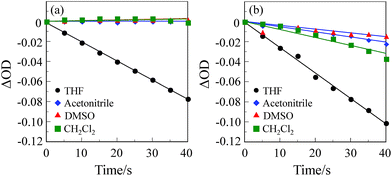
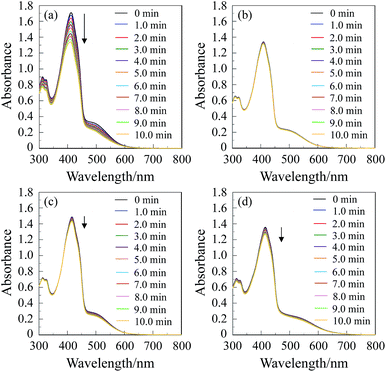
1O2 generation by (D–π–)2A pyrazinium dyes OEJ-1 and OEJ-2 in various solvents (THF, acetonitrile, DMSO and dichloromethane) was evaluated by monitoring the photoabsorption spectral change of the known 1O2 scavenger 1,3-diphenylisobenzofuran (DPBF) accompanied by the reaction of DPBF with the generated 1O2, that is, DPBF can trap 1O2 through its photooxidation.22 All the solvents were bubbled with air for 15 min. The air-saturated solution containing the dye (OEJ-1 or OEJ-2) and DPBF was irradiated with 509 nm (160 μW cm−2, see Table 1 for ε/M−1 cm−1@λabs = 509 nm) obtained by passage of xenon light through monochromator. The absorption band of DPBF at around 410 nm decreased with the increase in the photoirradiation time (Fig. 4 and 5), which indicate the reaction of DPBF with 1O2 generated upon the excitation of (D–π–)2A pyrazinium dyes. To gain insight into the effect of the solvent and the counter anion on the efficiency of DPBF photooxidation, the changes in optical density (ΔOD) of DPBF are plotted against the photoirradiation time (Fig. 6), and the slope (msl) is used to estimate the 1O2 quantum yield (ΦΔ) for OEJ-1 and OEJ-2. It was revealed that the msl value for OEJ-2 becomes steeper in the following order: DMSO (−0.4 × 10−3) < acetonitrile (−0.5 × 10−3) < dichloromethane (−0.8 × 10−3) < THF (−2.6 × 10−3), that is, the msl value in THF is larger than those in the other solvents. It was also found that the msl value of OEJ-2 is larger than that of OEJ-1 (−2.0 × 10−3 in THF). Consequently, this result indicates that THF is a favorable solvent for the (D–π–)2A pyrazinium dyes to present a high DPBF-oxidation efficiency compared with the other solvents. Moreover, the plots demonstrate that OUJ-2 bearing I− ion exhibits higher DPBF-oxidation efficiency than OUJ-1 bearing Br− ion. Thus, the ΦΔ values of OEJ-1 and OEJ-2 were estimated by the relative method using Rose Bengal (RB) (ΦΔ = 0.80) in methanol as the standard (Table 1). The ΦΔ value of OEJ-2 is 0.03, 0.05, 0.07 and 0.22 in DMSO, acetonitrile, dichloromethane and THF, respectively, which is in good agreement with the msl value. A higher ΦΔ value in THF is ascribable to that as for the (D–π–)2A pyrazinium dyes the ISC from 1S* to the 3S* may be facilitated by THF, although further study for the solvent effects on 1O2 generation is necessary to ensure the hypothesis. It is worth noting that the ΦΔ values of OEJ-2 in all the four solvents are higher than those of OEJ-1. Therefore, the high ΦΔ value of OEJ-2 relative to OEJ-1 is attributed to the fact that I− ion possesses superior heavy-atom effect rather than Br− ion, resulting in the facilitation of the ISC.In order to evaluate the photosensitizing ability of the (D–π–)2A pyrazinium dyes, the ln(Ct/C0) is plotted against the photoirradiation time, where Ct is a concentration of DPBF at the reaction time (t) and C0 is the initial concentration of DPBF before photoirradiation (Fig. 9). All the four solvents (THF, acetonitrile, DMSO and dichloromethane) were bubbled with air for 15 min. The air-saturated solution containing the dye (OEJ-1 or OEJ-2) and DPBF was irradiated with visible light (>510 nm, 6 mW cm−2) obtained by passage of xenon light through a 510 nm long path filter. The photoabsorption spectral changes for the photooxidation of DPBF using OEJ-1 and OEJ-2 under photoirradiation with the visible light in the four solvents are shown in Fig. 7 and 8, respectively. The ln(Ct/C0) decreased almost linearly with the increase in the photoirradiation time, although the linear relationship for the ln(Ct/C0) for OEJ-2 in dichloromethane become steeper after the photoirradiation for 5 min under this photoirradiation. Thus, this result indicates the ln(Ct/C0) bears a linear relationship with the photoirradiation time to provide the first-order rate constants (Kobs) for the photooxidation of DPBF using OEJ-1 and OEJ-2 as the photosensitizer (Table 1). The Kobs values for OEJ-2 are greater than those of OEJ-1, although the Kobs value for OEJ-2 in THF is lower than that of OEJ-1 due to the low photoabsorption property of OEJ-2 in THF. Interestingly, the plot of OEJ-2 in dichloromethane show a non-linear relationship, but the slope for OEJ-2 become steeper after 5 min of photoirradiation and the Kobs value for OEJ-2 in dichloromethane is greater than those in the other solvents. This interesting observation may be attributed to not only the bathochromic shift and broadening of absorption but also the enhancement of heavy-atom effect with the increase in the photoirradiation time in dichloromethane, that is, the significant specific solvatochromic behavior of the (D–π–)2A pyrazinium dye bearing I− ion. Therefore, this result demonstrates that OEJ-2 exhibits more efficient photosensitizing ability compared to OEJ-1, due to a superior heavy-atom effect of I− ion.
In addition, we performed an electron paramagnetic resonance (EPR) method with 2,2,6,6-tetramethyl-4-piperidone (4-oxo-TEMP) as the spin-trapping agent, which can react with 1O2 to produce 4-oxo-TEMPO as a stable nitroxide radical.14,23 When the air-saturated solution containing OEJ-2 and 4-oxo-TEMP was irradiated with visible light (>510 nm, 14 mW cm−2) obtained by passage of xenon light through a 510 nm long path filter, the ESR spectrum of 4-oxo-TEMPO was clearly observed as a characteristic 1:1:1 triplet (Fig. S6†). Moreover, to obtain the direct evidence of 1O2 generation by (D–π–)2A pyrazinium dyes, a phosphorescence spectrum of 1O2 was measured in air-saturated THF solution of OEJ-2. The phosphorescence maximum of 1O2 produced upon the excitation of OEJ-2 at 467 nm was clearly observed at around 1270 nm (Fig. S7†).6b,14,24 Consequently, this work demonstrated that (D–π–)2A pyrazinium dyes possess the ability to generate 1O2 under visible light irradiation.
Conclusions
(D–π–)2A pyrazinium dyes bearing a counter anion (X− = Br− or I−) which show specific solvatochromic behavior leading to the bathochromic shift of photoabsorption band in halogenated solvents, have been designed and developed as a photosensitizer possessing singlet oxygen (1O2) generation. This work demonstrated that the (D–π–)2A pyrazinium dyes possess the ability to generate 1O2 under visible light irradiation, due to the effective intersystem crossing (ISC) from the singlet excited state of the photosensitizer (1S*) to the triplet excited state (3S*) by the heavy-atom effect of the counter anion. It was found that the 1O2 quantum yield (ΦΔ) of OEJ-2 bearing I− ion is higher than that of OEJ-1 bearing Br− ion. Consequently, this result indicates that the high ΦΔ value of OEJ-2 relative to OEJ-1 is attributed to the fact that I− ion possesses superior heavy-atom effect rather than Br− ion, resulting in the facilitation of the ISC. Moreover, it was found that THF is a favorable solvent for the (D–π–)2A pyrazinium dyes to provide higher ΦΔ value compared with the polar solvents such as acetonitrile and DMSO. Thus, this result suggests that as for (D–π–)2A pyrazinium the ISC from 1S* to the 3S* may be facilitated by THF, although much effort for the solvent effects on 1O2 generation is necessary to ensure the hypothesis. Interestingly, the first-order rate constants (Kobs) for the photooxidation of DPBF using OEJ-2 in dichloromethane is greater than those in the other solvents, which is attributed to the bathochromic shift and broadening of absorption in dichloromethane, that is, the significant specific solvatochromic behavior of the (D–π–)2A pyrazinium dye bearing I− ion. Further study to gain greater insight into the effects of the molecular structure of pyrazinium dyes on the 1O2 generation efficiency is now in progress by developing the (D–π–)2A pyrazinium photosensitizers possessing strong photoabsorption property in body therapeutic window (650–900 nm) and water solubility.Experimental
General
Melting points were measured with a Yanaco micro melting point apparatus MP model. IR spectra were recorded on a SHIMADZU IRAffinity-1 spectrometer by ATR method. High-resolution mass spectral data were acquired on a Thermo Fisher Scientific LTQ Orbitrap XL. 1H NMR spectra were recorded on a Varian-400 (400 MHz) FT NMR spectrometer. Photoabsorption spectra were observed with a HITACHI U-2910 spectrophotometer. Cyclic voltammetry (CV) curves were recorded in and acetonitrile/Bu4NClO4 (0.1 M) solution with a three-electrode system consisting of Ag/Ag+ as reference electrode, Pt plate as working electrode, and Pt wire as counter electrode by using a Electrochemical Measurement System HZ-7000 (HOKUTO DENKO).Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 as eluent) to give OEJ-1 (0.078 g, yield 46%) as dark red solids; mp 152–153 °C; IR (ATR):
3 as eluent) to give OEJ-1 (0.078 g, yield 46%) as dark red solids; mp 152–153 °C; IR (ATR): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1624, 1591, 1526, 1487, 1445, 1427 cm−1; 1H NMR (400 MHz, DMSO-d6) δ = 0.83 (t, J = 7.4 Hz, 6H), 1.01 (t, J = 7.5 Hz, 3H), 1.19–1.26 (m, 4H), 1.46–1.52 (m, 2H), 1.65–1.73 (m, 4H), 2.07–2.14 (m, 2H), 4.35 (t, J = 7.0 Hz, 4H), 4.58 (t, J = 7.3 Hz, 2H), 6.89 (dd, J = 1.8 and 8.4 Hz, 2H), 7.04–7.09 (m, 12H), 7.18 (d, J = 1.7 Hz, 2H), 7.31–7.36 (m, 8H), 7.66 (dd, J = 1.5 and 8.3 Hz, 2H), 7.98 (d, J = 4.0 Hz, 2H), 8.06 (s, 2H), 8.10 (d, J = 8.4 Hz, 2H), 8.16 (d, J = 8.1 Hz, 2H), 8.25 (d, J = 4.0 Hz, 2H), 9.51 (s, 2H) ppm; 13C NMR (100 MHz, DMSO-d6) δ = 13.49, 13.71, 19.06, 19.75, 30.68, 32.23, 34.40, 41.80, 104.80, 106.26, 116.73, 117.48, 117.68, 120.58, 121.62, 122.90, 123.03, 123.59, 123.71, 129.38, 129.50, 136.45, 140.89, 140.95, 142.12, 146.34, 147.57, 147.63, 151.89, 152.04 ppm; HRMS (ESI): m/z (%): calcd for C72H65N6S2+ 1077.47066; found 1077.47144.
= 1624, 1591, 1526, 1487, 1445, 1427 cm−1; 1H NMR (400 MHz, DMSO-d6) δ = 0.83 (t, J = 7.4 Hz, 6H), 1.01 (t, J = 7.5 Hz, 3H), 1.19–1.26 (m, 4H), 1.46–1.52 (m, 2H), 1.65–1.73 (m, 4H), 2.07–2.14 (m, 2H), 4.35 (t, J = 7.0 Hz, 4H), 4.58 (t, J = 7.3 Hz, 2H), 6.89 (dd, J = 1.8 and 8.4 Hz, 2H), 7.04–7.09 (m, 12H), 7.18 (d, J = 1.7 Hz, 2H), 7.31–7.36 (m, 8H), 7.66 (dd, J = 1.5 and 8.3 Hz, 2H), 7.98 (d, J = 4.0 Hz, 2H), 8.06 (s, 2H), 8.10 (d, J = 8.4 Hz, 2H), 8.16 (d, J = 8.1 Hz, 2H), 8.25 (d, J = 4.0 Hz, 2H), 9.51 (s, 2H) ppm; 13C NMR (100 MHz, DMSO-d6) δ = 13.49, 13.71, 19.06, 19.75, 30.68, 32.23, 34.40, 41.80, 104.80, 106.26, 116.73, 117.48, 117.68, 120.58, 121.62, 122.90, 123.03, 123.59, 123.71, 129.38, 129.50, 136.45, 140.89, 140.95, 142.12, 146.34, 147.57, 147.63, 151.89, 152.04 ppm; HRMS (ESI): m/z (%): calcd for C72H65N6S2+ 1077.47066; found 1077.47144.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1624, 1591, 1528, 1487, 1445, 1425 cm−1; 1H NMR (400 MHz, DMSO-d6) δ = 0.83 (t, J = 7.5 Hz, 6H), 1.01 (t, J = 7.4 Hz, 3H), 1.18–1.27 (m, 4H), 1.44–1.52 (m, 2H), 1.65–1.73 (m, 4H), 2.06–2.14 (m, 2H), 4.35 (t, J = 7.0 Hz, 4H), 4.58 (t, J = 7.7 Hz, 2H), 6.89 (dd, J = 1.8 and 8.4 Hz, 2H), 7.04–7.09 (m, 12H), 7.18 (d, J = 1.7 Hz, 2H), 7.31–7.36 (m, 8H), 7.66 (dd, J = 1.4 and 8.1 Hz, 2H), 7.98 (d, J = 4.0 Hz, 2H), 8.06 (s, 2H), 8.10 (d, J = 8.4 Hz, 2H), 8.16 (d, J = 8.2 Hz, 2H), 8.25 (d, J = 4.0 Hz, 2H), 9.50 (s, 2H) ppm; 13C NMR (100 MHz, DMSO-d6) δ = 13.49, 13.71, 19.06, 19.75, 30.69, 32.22, 41.79, 104.81, 106.28, 116.75, 117.50, 117.68, 120.59, 121.61, 122.89, 123.04, 123.69, 129.31, 129.37, 129.50, 136.43, 140.89, 142.12, 146.33, 147.56, 151.90, 152.06 ppm (one aliphatic and two aromatic carbon signals were not observed owing to overlapping resonances); HRMS (ESI): m/z (%): calcd for C72H65N6S2+ 1077.47066; found 1077.47131.
= 1624, 1591, 1528, 1487, 1445, 1425 cm−1; 1H NMR (400 MHz, DMSO-d6) δ = 0.83 (t, J = 7.5 Hz, 6H), 1.01 (t, J = 7.4 Hz, 3H), 1.18–1.27 (m, 4H), 1.44–1.52 (m, 2H), 1.65–1.73 (m, 4H), 2.06–2.14 (m, 2H), 4.35 (t, J = 7.0 Hz, 4H), 4.58 (t, J = 7.7 Hz, 2H), 6.89 (dd, J = 1.8 and 8.4 Hz, 2H), 7.04–7.09 (m, 12H), 7.18 (d, J = 1.7 Hz, 2H), 7.31–7.36 (m, 8H), 7.66 (dd, J = 1.4 and 8.1 Hz, 2H), 7.98 (d, J = 4.0 Hz, 2H), 8.06 (s, 2H), 8.10 (d, J = 8.4 Hz, 2H), 8.16 (d, J = 8.2 Hz, 2H), 8.25 (d, J = 4.0 Hz, 2H), 9.50 (s, 2H) ppm; 13C NMR (100 MHz, DMSO-d6) δ = 13.49, 13.71, 19.06, 19.75, 30.69, 32.22, 41.79, 104.81, 106.28, 116.75, 117.50, 117.68, 120.59, 121.61, 122.89, 123.04, 123.69, 129.31, 129.37, 129.50, 136.43, 140.89, 142.12, 146.33, 147.56, 151.90, 152.06 ppm (one aliphatic and two aromatic carbon signals were not observed owing to overlapping resonances); HRMS (ESI): m/z (%): calcd for C72H65N6S2+ 1077.47066; found 1077.47131.
Evaluation of 1O2 quantum yield
Quantum yields (ΦΔ) for singlet oxygen (1O2) generation by (D–π–)2A pyrazinium dyes (OEJ-1 and OEJ-2) in various solvents (THF, acetonitrile, DMSO and dichloromethane) were evaluated by monitoring the photoabsorption spectral change of the known 1O2 scavenger 1,3-diphenylisobenzofuran (DPBF) accompanied by the reaction of DPBF with the generated 1O2, that is, DPBF can trap 1O2 through its photooxidation. All the solvents were bubbled with air for 15 min. The absorbance of DPBF was adjusted to around 1.0 in air-saturated solvent. Concentration of OEJ-1 or OEJ-2 was adjusted with an absorbance of 0.2–0.3 at the irradiation wavelength (509 nm). The air-saturated solution containing the photosensitizer (OEJ-1 or OEJ-2) and DPBF was irradiated with 509 nm (160 μW cm−2) obtained by passage of xenon light through monochromator. The photoabsorption spectral change of DPBF with the photoirradiation was monitored with an interval of 5 s up to 40 s. The absorption band of DPBF at around 410 nm decreased with the increase in the photoirradiation time. The changes in optical density (ΔOD) of DPBF are plotted against the photoirradiation time, and the slope is used to estimate the ΦΔ of OEJ-1 and OEJ-2. The ΦΔ of OEJ-1 and OEJ-2 was estimated by the relative method using Rose Bengal (RB) (ΦΔ = 0.80) in methanol as the standard. Therefore, the 1ΦΔ values were calculated according to the following eqn (1):| ΦΔsam = ΦΔref × [(msam/mref) × (Lref/Lsam)] | (1) |
Photosensitizing ability
Photosensitizing ability of the (D–π–)2A pyrazinium dyes (OEJ-1 and OEJ-2) in various solvents (THF, acetonitrile, DMSO and dichloromethane) was evaluated by plotting the ln(Ct/C0) against the photoirradiation time, where Ct is a concentration of DPBF at the reaction time (t) and C0 is the initial concentration of DPBF before photoirradiation. All the solvents were bubbled with air for 15 min. The air-saturated solution containing the photosensitizer (1 × 10−5 M for OEJ-1 and OEJ-2, 1 × 10−6 M for RB) and DPBF (5 × 10−5 M) was irradiated with visible light (>510 nm, 6 mW cm−2) obtained by passage of xenon light through a 510 nm long path filter. The absorbance of DPBF was adjusted to around 1.0 in air-saturated solvent. The photooxidation of DPBF with the photoirradiation was monitored by following the decrease in the photoabsorption at around 410 nm with an interval of 20 s up to 10 min. The concentration (Ct) of DPBF at the reaction time (t) was calculated based on Lambert–Beer law (ADPBF = εcl). The ln(Ct/C0) decreased almost linearly with the increase in the photoirradiation time due to the photooxidation of DPBF, that is, the slope was used to estimate the rate constants (Kobs).1O2 detection by EPR spin-trapping method with 4-oxo-TEMP
The EPR spectra were recorded on a JEOL JES-RE1X spectrometer under the following experimental conditions: temperature 298 K, microwave power 1 mW, microwave frequency 9.439 GHz, field modulation 0.2 mT at 100 kHz, and scan time 4 min. The air-saturated THF solution containing OEJ-2 (0.01 mM) as the photosensitizer and 4-oxo-TEMP (50 mM) as the spin-trapping agent was irradiated with visible light (>510 nm, 14 mW cm−2 for 30 min) obtained by passage of xenon light through a 510 nm long path filter. The ESR spectrum of 4-oxo-TEMPO which is formed by the reaction of 4-oxo-TEMP with 1O2, was clearly observed as a characteristic 1:1:1 triplet (Fig. S6†).Phosphorescence measurement of 1O2
Phosphorescence spectrum of 1O2 was recorded on a HORIBA NanoLog spectrometer equipped with a 450 W xenon lamp and a photomultiplier tube (NIR-PMT R5509-43 liquid nitrogen configurations, Hamamatsu photonics). The phosphorescence maximum of 1O2 produced upon the excitation of OEJ-2 (0.07 mM THF solution) at 467 nm was clearly observed at around 1270 nm (Fig. S7†).Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 15H03859 and by Electric Technology Research Foundation of Chugoku. We would also like to thank Dr Yasushi Nakata of HORIBA, Ltd. for support with phosphorescence measurement of singlet oxygen.References
- J. F. Lovell, T. W. B. Liu, J. Chen and G. Zheng, Chem. Rev., 2010, 110, 2839 CrossRef CAS PubMed.
- M. C. DeRosa and R. J. Crutchley, Coord. Chem. Rev., 2002, 233–234, 351 CrossRef CAS.
- M. Pawlicki, H. A. Collins, R. G. Denning and H. L. Anderson, Angew. Chem., Int. Ed., 2009, 48, 3244 ( Angew. Chem. , 2009 , 121 , 3292 ) CrossRef CAS PubMed.
- K. A. Leonard, M. I. Nelen, L. T. Anderson, S. L. Gibson, R. Hilf and M. R. Detty, J. Med. Chem., 1999, 42, 3942 CrossRef CAS PubMed.
- F. Ronzani, A. Trivella, E. Arzoumanian, S. Blanc, M. Sarakha, C. Richard, E. Oliveros and S. Lecombe, Photochem. Photobiol. Sci., 2013, 12, 2160 CAS.
- (a) R. Schmidt and E. Afshari, J. Phys. Chem., 1990, 94, 4377 CrossRef CAS; (b) M. Mirenda, C. A. Strassert, L. E. Dicelio and E. S. Román, ACS Appl. Mater. Interfaces, 2010, 2, 1556 CrossRef CAS PubMed.
- (a) J. P. Belair, C. J. Ziegler, C. S. Rajesh and D. A. Modarelli, J. Phys. Chem. A, 2002, 106, 6445 CrossRef CAS; (b) P. C. Lo, J. D. Huang, D. Y. Y. Cheng, E. Y. M. Chan, W. P. Fong, W. H. Ko and D. K. P. Ng, Chem.–Eur. J., 2004, 10, 4831 CrossRef CAS PubMed; (c) A. Karotki, M. Khurana, J. R. Lepock and B. C. Wilson, Photochem. Photobiol., 2006, 82, 443 CrossRef CAS PubMed; (d) L. Delanaye, M. A. Bahri, F. Tfibel, M.-P. Fontaine-Aupart, A. Mouithys-Mickalad, B. Heine, J. Piette and M. Hoebeke, Photochem. Photobiol. Sci., 2006, 5, 317 RSC; (e) M. Morone, L. Beverina, A. Abbotto, F. Silvestri, E. Collini, C. Ferrante, R. Bozio and G. A. Pagani, Org. Lett., 2006, 8, 2719 CrossRef CAS PubMed; (f) M. Khurana, H. A. Collins, A. Karotki, H. L. Anderson, D. T. Cramb and B. C. Wilson, Photochem. Photobiol., 2007, 84, 1441 CrossRef PubMed.
- (a) A. P. Thomas, P. S. S. Babu, S. A. Nair, S. Ramakrishnan, D. Ramaiah, T. K. Chandrashekar, A. Srinivasan and M. R. Pillai, J. Med. Chem., 2012, 55, 5110 CrossRef CAS PubMed; (b) K. Hirakawa, Y. Nishimura, T. Arai and S. Okazaki, J. Phys. Chem. B, 2013, 117, 13490 CrossRef CAS PubMed; (c) H. Horiuchi, M. Hosaka, H. Mashio, M. Terata, S. Ishida, S. Kyushin, T. Okutsu, T. Takeuchi and H. Hiratsuka, Chem.–Eur. J., 2014, 20, 6054 CrossRef CAS PubMed; (d) Q. Yu, E. M. Rodriguez, R. Naccache, P. Forgione, G. Lamoureux, F. Sanz-Rodriguez, D. Scheglman and J. A. Capobianco, Chem. Commun., 2014, 50, 12150 RSC; (e) D. Yao, V. Hugues, M. Blanchard-Desce, O. Mongin, C. O. Paul-Roth and F. Paul, New J. Chem., 2015, 39, 7730 RSC; (f) J. Schmitt, V. Heitz, A. Sour, F. Bolze, H. Ftouni, J.-F. Nicoud, L. Flamigni and B. Ventura, Angew. Chem., Int. Ed., 2015, 54, 169 ( Angew. Chem. , 2015 , 127 , 171 ) CrossRef CAS PubMed.
- (a) X. J. Jiang, P. C. Lo, Y. M. Tsang, S. L. Yeung, W. P. Fong and D. K. P. Ng, Chem.–Eur. J., 2010, 16, 4777 CrossRef CAS PubMed; (b) M. R. Ke, S. L. Yeung, W. P. Fong, D. K. P. Ng and P. C. Lo, Chem.–Eur. J., 2012, 18, 4225 CrossRef CAS PubMed; (c) A. R. Karimi and A. Khodadadi, Tetrahedron Lett., 2012, 53, 5223 CrossRef CAS; (d) S. G. Kimani, T. A. Shmigol, S. Hammond, J. B. Phillips, J. I. Bruce, A. J. MacRobert, M. V. Malakhov and J. P. Golding, Photochem. Photobiol., 2013, 89, 139 CrossRef CAS PubMed.
- (a) A. Gorman, J. Killoran, C. O'Shea, T. Kenna, W. M. Gallagher and D. F. O'Shea, J. Am. Chem. Soc., 2004, 126, 10619 CrossRef CAS PubMed; (b) T. Yogo, Y. Urano, Y. Ishitsuka, F. Maniwaa and T. Nagano, J. Am. Chem. Soc., 2005, 127, 12162 CrossRef CAS PubMed; (c) S. O. McDonnell, M. J. Hall, L. T. Allen, A. Byrne, W. M. Gallagher and D. F. O'Shea, J. Am. Chem. Soc., 2005, 127, 16360 CrossRef CAS PubMed; (d) M. J. Hall, L. T. Allen and D. F. O'Shea, Org. Biomol. Chem., 2006, 4, 776 RSC; (e) J. Killoran and D. F. O'Shea, Chem. Commun., 2006, 1503 RSC.
- (a) N. Adarsh, R. R. Avirah and D. Ramaiah, Org. Lett., 2010, 12, 5720 CrossRef CAS PubMed; (b) Y. Cakmak, S. Kolemen, S. Duman, Y. Dede, Y. Dolen, B. Kilic, Z. Kostereli, L. T. Yildirim, A. L. Dogan, D. Guc and E. U. Akkaya, Angew. Chem., Int. Ed., 2011, 50, 11937 ( Angew. Chem. , 2011 , 123 , 12143 ) CrossRef CAS PubMed; (c) S. G. Awuah, J. Polreis, V. Biradar and Y. You, Org. Lett., 2011, 13, 3884 CrossRef CAS PubMed; (d) N. Adarsh, M. Shanmugasundaram, R. R. Avirah and D. Ramaiah, Chem.–Eur. J., 2012, 18, 12655 CrossRef CAS PubMed.
- (a) Y. C. Yang, Q. L. Guo, H. C. Chen, Z. K. Zhou, Z. J. Guo and Z. Shen, Chem. Commun., 2013, 49, 3940 RSC; (b) W. Li, L. Li, H. Xiao, R. Qi, Y. Huang, Z. Xie, X. Jing and H. Zhang, RSC Adv., 2013, 3, 13417 RSC; (c) L. Huang, X. Cui, B. Therrien and J. Zhao, Chem.–Eur. J., 2013, 19, 17472 CrossRef CAS PubMed; (d) G. Awuah, S. K. Das, F. D'Souza and Y. You, Chem.–Asian J., 2013, 8, 3123 CrossRef PubMed; (e) S. Guo, L. Ma, J. Zhao, B. Küçüköz, A. Karatay, M. Hayvali, H. G. Yaglioglu and A. Elmali, Chem. Sci., 2014, 5, 489 RSC; (f) Y.-C. Lai and C.-C. Chang, J. Mater. Chem. C, 2014, 2, 1576 RSC; (g) R. L. Watley, S. G. Awuah, M. Bio, R. Cantu, H. B. Gobeze, V. N. Nesterov, S. K. Das, F. D'Souza and Y. You, Chem.–Asian J., 2015, 10, 1335 CrossRef CAS PubMed.
- (a) J. W. Arbogast and C. S. Foote, J. Am. Chem. Soc., 1991, 113, 8886 CrossRef CAS; (b) J. W. Arbogast, A. P. Darmanyan, C. S. Foote, F. N. Diederich, R. L. Whetten, Y. Rubin, M. M. Alvarez and S. J. Anz, J. Phys. Chem., 1991, 95, 11 CrossRef CAS; (c) A. Ikeda, T. Sato, K. Kitamura, K. Nishiguchi, Y. Sasaki, J. Kikuchi, T. Ogawa, K. Yogo and T. Takeya, Org. Biomol. Chem., 2005, 3, 2907 RSC; (d) P. Mroz, G. P. Tegos, H. Gali, T. Wharton, T. Sarna and M. R. Hamblin, Photochem. Photobiol. Sci., 2007, 6, 1139 RSC.
- Y. Yamakoshi, N. Umezawa, A. Ryu, K. Arakane, N. Miyata, Y. Goda, T. Masumizu and T. Nagano, J. Am. Chem. Soc., 2003, 125, 12803 CrossRef CAS PubMed.
- W. Wu, J. Sun, X. Cui and J. Zhao, J. Mater. Chem. C, 2013, 1, 4577 RSC.
- (a) R. Gao, D. G. Ho, B. Hernandez, M. Selke, D. Murphy, P. I. Djurovich and M. E. Thompson, J. Am. Chem. Soc., 2002, 124, 14828 CrossRef CAS PubMed; (b) P. I. Djurovich, D. Murphy, M. E. Thompson, B. Hernandez, R. Gao, P. L. Hunt and M. Selke, Dalton Trans., 2007, 3763 RSC; (c) S. Takizawa, R. Aboshi and S. Murata, Photochem. Photobiol. Sci., 2011, 10, 895 RSC.
- (a) J. C. Koziar and D. O. Cowan, Acc. Chem. Res., 1978, 11, 334 CrossRef CAS; (b) C. Schweitzer and R. Schmidt, Chem. Rev., 2003, 103, 1685 CrossRef CAS PubMed.
- (a) F. Ronzani, E. Arzoumanian, S. Blanc, P. Bordat, T. Pigot, C. Cugnet, E. Oliveros, M. Sarakha, C. Richard and S. Lacombe, Phys. Chem. Chem. Phys., 2013, 15, 17219 RSC; (b) D. Huand, J. Sun, L. Ma, C. Zhang and J. Zhao, Photochem. Photobiol. Sci., 2013, 12, 872 RSC; (c) S. Cobo, F. Lafolet, E. Saint-Aman, C. Philouze, C. Bucher, S. Silvi, A. Credi and G. Royal, Chem. Commun., 2015, 51, 13886 RSC.
- Y. Ooyama, K. Uenaka, Y. Harima and J. Ohshita, RSC Adv., 2014, 4, 30225 RSC.
- (a) Y. Ooyama, R. Asada, S. Inoue, I. Imae, K. Komaguchi and Y. Harima, New J. Chem., 2009, 33, 2311 RSC; (b) Y. Ooyama, K. Kushimoto, Y. Oda, D. Tokita, N. Yamaguchi, S. Inoue, T. Nagano, Y. Harima and J. Ohshita, Tetrahedron, 2012, 68, 8577 CrossRef CAS; (c) Y. Ooyama, Y. Oda, T. Mizumo and J. Ohshita, Tetrahedron, 2013, 69, 1755 CrossRef CAS; (d) Y. Ooyama, Y. Oda, T. Mizumo, Y. Harima and J. Ohshita, Eur. J. Org. Chem., 2013, 4533 CrossRef CAS.
- Both the geometry optimization and energy calculation were performed by employing the density functional theory (DFT), at the level of B3LYP/6-31G(d,p) on the Gaussian09 program package ( M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2009 Search PubMed).
- K. Golinick and A. Griesbeck, Tetrahedron, 1985, 41, 2057 CrossRef.
- S. Oriana, S. Aroua, J. O. B. Söllner, X.-J. Ma, Y. Iwamoto and Y. Yamakoshi, Chem. Commun., 2013, 49, 9302 RSC.
- (a) H. Horiuchi, T. Kameya, M. Hosaka, K. Yoshimura, S. Kyushin, H. Matsumoto, T. Okutsu, T. Takeuchi and H. Hiratsuka, J. Photochem. Photobiol., A, 2011, 221, 98 CrossRef CAS; (b) K. Hirakawa, N. Fukunaga, Y. Nishimura, T. Arai and S. Okazaki, Bioorg. Med. Chem. Lett., 2013, 23, 2704 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26647e |
| This journal is © The Royal Society of Chemistry 2016 |