Multifunctional single-drug loaded nanoparticles for enhanced cancer treatment with low toxicity in vivo†
Abstract
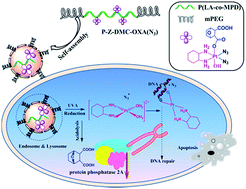
Platinum(II) complexes are used for treating over half of cancers in the clinic. However, their application is accompanied with acquired resistance and severe toxic side effects. To address these challenges, a multifunctional single drug constructed from a photo-activated oxaliplatin(IV) complex and DMC was conjugated to polymer to form multifunctional single-drug-loaded nanoparticles. When the nanoparticles are internalized by cancer cells via endocytosis, active oxaliplatin(II) can be released upon UVA irradiation to attack DNA, while DMC will be hydrolyzed subsequently from the polymer chain within the intracellular environment to block DNA repair by PP2A inhibition. The multifunctional single-drug-loaded nanoparticles exhibited enhanced anti-cancer efficacy and decreased toxicity compared to oxaliplatin(II) in vitro and in vivo. This enhanced cancer treatment strategy could have potential clinical use in the near future.

 Please wait while we load your content...
Please wait while we load your content...