Comparative study of simultaneous saccharification and fermentation byproducts from sugarcane bagasse using steam explosion, alkaline hydrogen peroxide and organosolv pretreatments
Yanzhi Youa,
Shujuan Yanga,
Lingxi Bub,
Jianxin Jiang*a and
Dafeng Sun*c
aDepartment of Chemistry and Chemical Engineering, MOE Engineering Research Center of Forestry Biomass Materials and Bioenergy, Beijing Forestry University, Beijing, 100083, China. E-mail: jiangjx@bjfu.edu.cn; sdafeng@163.com
bState Grid Energy Conservation Service Ltd, Beijing Biomass Energy Technology Center, Beijing, 100052, China. E-mail: bulingxi@sgcs.sgcc.com.cn; Fax: +86-10-60617615
cNanjing Institute for the Comprehensive Utilization of Wild Plant, Nanjing, 210042, China
First published on 15th January 2016
Abstract
Byproducts of simultaneous saccharification and fermentation (SSF) from sugarcane bagasse using steam explosion (SE, 190 °C for 10 min and 210 °C for 5 min) and green liquor (GL) combined with hydrogen peroxide (GL–H2O2) or ethanol (GL–ethanol) pretreatments were compared. Results showed that SE pretreatments did not result in lactic acid because a majority of the glucose was consumed for yeast growth and ethanol production, and the ethanol yield of 93.86% (of the theoretical) at 190 °C for 10 min and 94.33% at 210 °C for 5 min were achieved. Most of the hemicelluloses were removed and more acetyl groups were generated after the SE pretreatment, so it always had the highest amount of acetic acid (1.22 g L−1 at 190 °C for 10 min and 1.38 g L−1 at 210 °C for 5 min). GL–ethanol pretreatment resulted in an ethanol yield of 80.56%. However, the existence of reactive lignin from organic solvent produced high amount of byproducts, especially for the maximum glycerol contents (0.818 g L−1). Deficient lignin removal and sugar degradation with GL–H2O2 pretreatment led to the lowest ethanol yield of 23.23%, which may be because some inhibitors generated from GL–H2O2 pretreatment affected hydrolysis efficiency and yeast activity in the SSF process.
1. Introduction
Lignocellulosic biomass is the most abundant renewable resource on Earth. It has the potential to partly replace fossil-based resources (e.g., coal, crude oil) for the production of fuels and chemicals. This would reduce the levels of greenhouse gas emissions and alleviate the pressure on the depletion of the fossil-based non-renewable resources.1 Among the various agricultural and industrial residues, sugarcane bagasse (SCB) is one of the most abundant lignocellulosic resources,2,3 especially in tropical countries. The enormous utilization of sugarcane for sugar and ethanol production in tropical countries, such as Brazil, China and India, generates >500 Mt of bagasse as byproduct every year.3 However, SCB is normally burned in industries to supply all the energy required in the process. If, instead, it is used for ethanol production, much more ethanol would be produced from each hectare of sugarcane processed.4Bioethanol production from lignocellulosic biomass, using Saccharomyces cerevisiae, comprises the hydrolysis of cellulose and sugar fermentation.5 However, due to the recalcitrance of lignocellulosic biomass, efficient production of fermentable sugars requires pretreatment to deconstruct biomass and hydrolysis of pretreated biomass with enzymes to obtain fermentable sugars as well as prevent sugar degradation and minimize the consequent formation of toxic derivatives.6 To date, a variety of pretreatment methods, including physical, chemical, biological and combined methods, have been studied.7
Pretreatment methods such as fiber explosion (steam, ammonia, and CO2) could efficiently remove hemicelluloses to increase the accessibility of cellulose.8 Steam explosion (SE) pretreatment is one of the most attractive pretreatment processes owing to its low chemical usage and energy consumption.8 It disrupts the lignin barrier and facilitates the contact of the enzyme with cellulose by removing the hemicelluloses.9 Alkaline hydrogen peroxide has been proven to be a good choice for the pretreatment of lignocellulosic biomass,10–12 because it leads to high glucose yields and can be used under conditions of moderate temperature and pressure without adding acid, which leads to the formation of minor inhibitors.13 It is a typical environment-friendly agent used for delignification in wood pulping processes,14 and leaves no residues in the biomass, because it degrades in oxygen and water, thus minimizing the need for waste treatment. Organosolv pretreatment has been considered as an efficient pretreatment method for lignocellulosic biomass. Organosolv pretreatment generally leads to significant delignification and/or improved fiber porosity, thereby improving cellulose accessibility to cellulase.15,16 In addition, it can improve the efficiency of the whole process through the utilization of byproducts.17,18 Among these organosolv pretreatments, ethanol pretreatment has been conducted incorporating acid catalysts to eliminate the extreme temperature and pressure conditions required for the uncatalyzed pretreatment systems.19 It is also considered as the preferable method because of the lower toxicity and use of low boiling point solvents, which can be easily recovered by distillation. Green liquor (GL) is a mixture of sodium carbonate and sodium hydroxide, which is produced from pulping processes. Recently, pretreatment combined with GL has been developed as a better method to increase the enzymatic digestibility of biomass, retaining maximum levels of polysaccharide in the substrate for enzymatic hydrolysis.20
However, there are also some limitations produced from pretreatment; it, at least partially, degrades hemicellulose-derived sugars and transforms lignin compounds into chemicals, such as weak acids (lactic and acetic acid) and glycerol, that can inhibit the downstream process.9 The weak organic acids, namely, lactic and acetic, are of utmost concern to fuel alcohol producers because they are potential inhibitors of yeast growth and metabolism.21 The effect of glycerol byproduct on ethanol production during the fermentation of Saccharomyces cerevisiae is significant, because the generation of glycerol consumes at least 4% of the carbon source for the fermentation.21 Glycerol is obtained under the osmotic pressure changes and low oxidation–reduction potential.22 It is also possible that glycerol may act as a glucose analogue, resulting in end product inhibition of cellulase activity.23 Most of the research conducted to date on the inhibitory effects of lactic acid and acetic acid on yeast growth and metabolism have focused primarily on the response of yeast to each acid individually. Independently, lactic and acetic acids have been shown to cause increased lag times, decreased growth rates, reduced biomass yields and even cell death24 in S. cerevisiae cultures.
In this study, SCB was pretreated with SE at 190 °C for 10 min and 210 °C for 5 min or by using GL combined with hydrogen peroxide (GL–H2O2) and ethanol (GL–ethanol). Then, the pretreated materials were subjected to a process known as simultaneous saccharification and fermentation (SSF).25 The primary objective of the current study, therefore, was to examine the effects of pretreatment methods on the production of ethanol, lactic acid, acetic acid, and glycerol from SCB in the SSF. The raw material was used for comparison.
2. Experimental section
2.1. Materials
SCB were used as feedstock, which were kindly provided by Guitang Corporation (Guangxi, China). The dry SCB was divided into two parts. One was ground and screened with 40 mesh sieves, collected in sealed plastic bags, and stored at room temperature for further GL pretreatment. The other was steam pretreated. The GL was supplied by Chenming Group (Shandong, China). The supernatant was obtained for utilization after precipitating overnight. The main components of GL were sodium carbonate (75.2 ± 0.25 g L−1) and sodium hydroxide (23.04 ± 0.25 g L−1). There were also other metal elements in GL such as iron (1.14 ± 0.08 g L−1) and calcium (0.39 ± 0.03 g L−1).8 Anthraquinone with 1% w/w dry matter (AQ, Sigma Co., St Louis, MO, USA) was used in GL–ethanol pretreatment. All the chemicals used in this study were of analytical grade. The main components of raw and pretreated materials were analyzed for the following process.2.2. Steam explosion (SE) pretreatment
The SCB was impregnated with 4% concentrated sulfuric acid at a ratio of solid to liquid of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 for 1 h. Then, SE was performed at 190 °C for 10 min and 210 °C for 5 min with final moisture content of 55 wt% in a high pressure batch reactor (20 L) located in National Power Grid (Beijing). A portion of the cellulignin after SE was stored at 4 °C for subsequent chemical analyses and SSF process, and the liquid was reserved and pretreated with or without acid hydrolysis for the determination of sugars (mono-meric and oligomeric fraction) by high performance liquid chromatography (HPLC).
10 for 1 h. Then, SE was performed at 190 °C for 10 min and 210 °C for 5 min with final moisture content of 55 wt% in a high pressure batch reactor (20 L) located in National Power Grid (Beijing). A portion of the cellulignin after SE was stored at 4 °C for subsequent chemical analyses and SSF process, and the liquid was reserved and pretreated with or without acid hydrolysis for the determination of sugars (mono-meric and oligomeric fraction) by high performance liquid chromatography (HPLC).
2.3. Green liquor (GL) combined with ethanol (GL–ethanol) and hydrogen peroxide (GL–H2O2) pretreatment
GL–ethanol pretreatment was carried out in a polytetrafluoroethylene (PTFE) reactor with a total volume of 200 mL according to a previous optimized study.8 10 g of dry SCB was pretreated with 1.5 mL of GL per g-dry matter (DS) at 140 °C for 3 h with the solid/liquid ratio (w/v) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The liquid was a 50
10. The liquid was a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) ethanol
50 (v/v) ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixture. The system (PTFE reactor + stainless steel tank) was placed in a chamber equipped with a shaft, where the PTFE reactor was fixed well with a large stainless steel tank. The system was heated at an average rate of 5 °C min−1 and rotated at 100 rpm to reach a desired temperature of 140 °C. The system was rapidly cooled with tap-water after the pretreatment. The pretreated SCB was obtained by filtration prior to washing with 200 mL of ethanol–water mixture (50
water mixture. The system (PTFE reactor + stainless steel tank) was placed in a chamber equipped with a shaft, where the PTFE reactor was fixed well with a large stainless steel tank. The system was heated at an average rate of 5 °C min−1 and rotated at 100 rpm to reach a desired temperature of 140 °C. The system was rapidly cooled with tap-water after the pretreatment. The pretreated SCB was obtained by filtration prior to washing with 200 mL of ethanol–water mixture (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v). Then, the solid fraction thus obtained was washed with distilled water until neutral pH was obtained and dried in an oven at 103 ± 2 °C.
50, v/v). Then, the solid fraction thus obtained was washed with distilled water until neutral pH was obtained and dried in an oven at 103 ± 2 °C.
GL–H2O2 pretreatment was carried out in a PTFE reactor at 80 °C, which was rotated at 100 rpm for 3 h according to a previous optimized study.8 The SCB was slurried in water (5%, w/v) containing the desired amount of H2O2 (0.6 g per g-dry substrate (DS)) and 1% (w/w) ethylenediaminetetraacetic acid (EDTA, 98%, Sinopharm Chemical reagent Beijing Co., Ltd). After the pretreatment, the pretreated SCB was collected by filtration and washed with distilled water until the pH was neutral.
2.4. Microorganisms, inoculums and enzyme preparation
The microorganism, Saccharomyces cerevisiae, in the form of dry yeast was purchased from Angel Yeast Company (YiChang, China). Dry yeast was activated in a 2% glucose solution at 36 °C for 15 min and then at 34 °C for 1 h before SSF. Cellulolytic enzymes were Celluclast 1.5L with a cellulase activity of 75 FPU mL−1 and Novozym 188 with a β-glucosidase activity of 43.9 IU mL−1, and both were kindly donated by Novozymes A/S (Bagsvaerd, Denmark).2.5. Simultaneous saccharification and fermentation (SSF)
The SSF experiments were performed under nonsterile conditions in a 100 mL conical flask with a working weight of 60 g, and each flask was equipped with a loop trap containing sterile glycerol for the discharge of carbon dioxide, which could reduce the loss of ethanol. The amount of the enzymes Celluclast 1.5 L and Novozym 188 was 30 FPU per g-cellulose and 37.5 IU per g-cellulose, respectively. The concentration of insoluble fibrous material was 5% (w/v) on the basis of dry material in all the experiments. The initial inoculum concentration of yeast was about 5 g L−1. Fermentation with full medium (yeast powder, 1 g L−1; (NH4)2HPO4, 0.5 g L−1; MgSO4·7H2O, 0.25 g L−1) and fermentation with raw material as control cases were carried out. In each experiment, SCB in the conical flask and nutrients were separately sterilized (121 °C for 20 min). The fermentation was conducted in an air bath shaker at a speed of 120 rpm at 38 °C for 120 h.2.6. Analysis
The cellulose and hemicellulose contents of the samples were determined by standard analytical methods. Acid-insoluble lignin of SCB was determined by the TAPPI method (TAPPI T222 om-06 2006). The Klason lignin content was taken as the ash free residue after acid hydrolysis. A muffle furnace was used at 600 °C to determine the percentage of total ash according to the residue weight.26Ethanol, glycerol, lactic acid and other byproducts were analyzed by HPLC (Waters 2695e, USA) using an Aminex HPX-87H column (300 × 7.8 mm; Bio-Rad Laboratories, USA) at 65 °C and a refractive index detection detector at 35 °C. The injection volume of the sample was 10 μL, and 5 mM sulfuric acid was used as the eluent at a flow rate of 0.6 mL min−1. The ethanol yield was calculated assuming that 1 g of cellulose present in the liquid theoretically gave 0.568 g of ethanol, and it is expressed as the percentage of the theoretical yield based on SCB and cellulose. Assays were performed in two repeated experiments, and the mean values are presented.
3. Results and discussion
3.1. Comparison of pretreatments on the chemical composition and ethanol yield
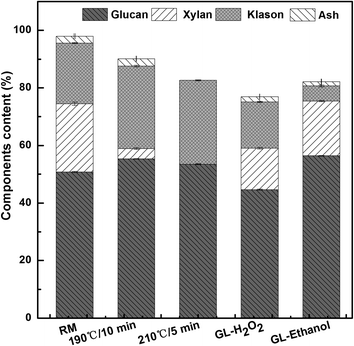
The effects of pretreatment methods on various component and ethanol yields of SCB are shown in Fig. 1 and 2. For the raw and pretreated materials, glucan was the dominant component, followed by lignin and xylan. These results are similar to those previously reported.27,28 These values are important in terms of biorefinery principle. Lignocellulosic materials with high glucan and xylan contents are preferable candidates for bio-ethanol production.2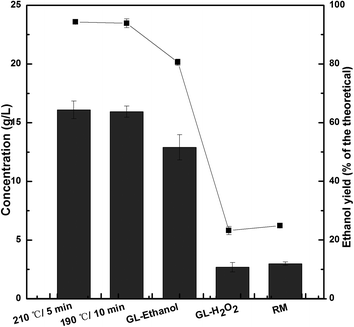 | ||
| Fig. 2 Comparison of pretreatments on the final ethanol concentration and yield from 5% (w/v) pretreated or raw material (RM) during the SSF process at 38 °C with initial pH = 5.5 for 120 h. | ||
The SE pretreatment removes hemicelluloses and also some lignin depending on the extent of the treatment. This has been previously described by Martín-Sampedro et al., who have found that hemicelluloses are removed by hydrolysis and lignin softens in the heat and slowly depolymerizes.29 The results showed that 85.02% and 100% of hemicelluloses were degraded by SE pretreatment at 190 °C for 10 min and 210 °C for 5 min, respectively, and the highest ethanol yield of 93.86% (15.93 g L−1) was reached at 190 °C for 10 min and 94.33% (16.08 g L−1) was reached at 210 °C for 5 min through SSF for 120 h, respectively (Fig. 1 and 2), indicating that the structure became loose and the surface area of the fibers increased because of delignification and enzymatic hydrolysis after the SE pretreatment.
For the GL pretreatment process, the content of lignin decreased sharply from 21.12% to 5.26%, and the preferable amount of glucan increased from 50.74% to 56.39% during GL–ethanol (1.5 mL GL per g-DS) pretreatment with the use of anthraquinone (AQ) (1%, w/w), which is better than the results obtained using two SE pretreatment methods. Researchers have proved that AQ used in the soda pulping process improves delignification and reduces carbohydrate decomposition.30,31 However, the final ethanol yield of 80.56% (12.90 g L−1) with the GL–ethanol pretreatment was lower than that with two SE pretreatments in the SSF (Fig. 2). It was likely that the release of hemicelluloses after SE pretreatment increased the specific area of the pretreated biomass.32 However, the existence of reactive lignin resulting from use of an organic solvent led to some inhibitor production in the case of GL–ethanol pretreatment.
Conversely, only 24.05% of lignin removal was observed during GL–H2O2 (0.6 g H2O2 per g-DS) pretreatment. The maximum ethanol concentration of 2.69 g L−1 (23.23% of the theoretical amount) was achieved, much less than that form GL–ethanol pretreatment (Fig. 2). These results indicated that SCB pretreated with GL–H2O2 had a bigger contact angle, implying that this substrate was more hydrophobic compared with GL–ethanol pretreated SCB.33 A previous study showed that the glucose yield after 72 h enzymatic hydrolysis with 0.6 g of H2O2 per g-DS during GL–H2O2 pretreatment was considerably lower than that with 1.5 mL of GL per g-DS during GL–ethanol pretreatment.8 Therefore, it was necessary to reduce the dosage of H2O2 during the alkaline pretreatment to minimize the glucan degradation for the untreated SCB.
3.2. Comparison of pretreatments for lactic acid production
In the SSF process, sugars were reported to be converted to ethanol, as well as to byproducts, with the extension of the fermentation time and consumption of nutrients in the substrate.34,35 These byproducts exhibited a negative or positive influence on ethanol production.It has been claimed that SSF is much less sensitive to contamination than separate hydrolysis and fermentation processes.36 A previous study showed that it was difficult to completely avoid infection in SSF.37
The bioconversion of lignocellulosic biomass to lactic acid occurs in two steps. The cellulose is first depolymerised by cellulase to produce glucose, which is subsequently fermented to produce lactic acid.38 Lactic acid is produced by contaminating lactic acid bacteria as a result of carbohydrate metabolism.39 Fig. 3 shows the effects of the different pretreatments on the concentration of lactic acid during the SSF process of 5% (w/v) pretreated or raw material (RM) at 38 °C with initial pH = 5.5. In Fig. 3, similar low concentration profiles of lactic acid from two steam-exploded materials are observed in the total ethanol fermentation compared with the untreated material, indicating that a majority of the glucose was consumed for yeast growth as soon as it was released,9 thus only a small part of the glucose was consumed for lactic acid production. However, no lactic acid was detected in the initial time of 48 h of SSF, and there was a sharp increase in output of lactic acid for the GL–H2O2 pretreatment in the following time, resulting in the highest concentration (1.72 g L−1) corresponding to the amount of raw material at 120 h compared with other two pretreatments (Fig. 3). These data showed that the RM and GL–H2O2 pretreated materials were more likely to be infected with lactic acid bacteria, thus the ethanol yield of the GL–H2O2 pretreated material was close to that of the raw material with 120 h SSF (Fig. 2). Moreover, SSF was better than a separate hydrolysis process in exhibiting a much greater sensitivity to infection, even when a pure culture of yeast was used.37 This indicated that the yeast facilitates the growth of lactic acid bacteria, which has been reported in the literature.40,41 In contrast, lactic acid concentration of the GL–ethanol pretreatment was found to be 1.17 g L−1 after a 72 h SSF (Fig. 3). This indicated that small adherent reactive lignin might generate some inhibitors and could influence the activity of lactic acid bacteria and the yield of lactic acid in the case of GL–ethanol pretreatment. These results implied that the steam explosion pretreatments for ethanol production were better than the GL pretreatments, as indicated in Fig. 2.
 | ||
| Fig. 3 Comparison of pretreatments on the concentration of lactic acid from 5% (w/v) pretreated or raw material (RM) during the SSF process at 38 °C with initial pH = 5.5 for 120 h. | ||
3.3. Comparison of the pretreatments on acetic acid production
Acetic acid is primarily produced by acetic acid bacteria and/or lactic acid bacteria. Acetic acid production is not always the result of bacterial contamination, because minor concentrations are generated by Saccharomyces cerevisiae during fermentation.36 Fig. 4 shows the effects of the different pretreatments on the concentration of acetic acid during the SSF process of 5% (w/v) pretreated or raw material (RM) at 38 °C with initial pH = 5.5. As indicated in Fig. 4, similar concentration profiles of acetic acid (1.22, 1.38 and 1.18 g L−1) of the steam-exploded (190 °C for 10 min and 210 °C for 5 min) and GL–ethanol pretreated bagasse are still observed throughout the entire SSF, and the acetic acid concentrations at 190 °C for 10 min and 210 °C for 5 min steam-exploded bagasse are higher than that of GL–ethanol pretreated one, indicating that most of the hemicelluloses were removed and more acetyl groups were generated after the steam explosion pretreatment. Previous studies also have reported that acetic acid is derived from hemicellulose degradation.42 In contrast, there was a leveling off in the fermentation of the GL–H2O2 pretreated material after 72 h, in which the acetic acid concentration was similar to that of the raw material. This indicated that the formation of ethanol and acetic acid in the fermentation period had reached a balance.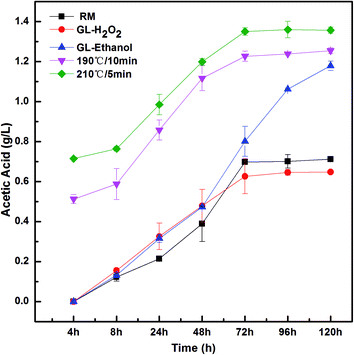 | ||
| Fig. 4 Comparison of pretreatments for the concentration of acetic acid from 5% (w/v) pretreated or raw material (RM) during the SSF process at 38 °C with initial pH = 5.5 for 120 h. | ||
On the other hand, researchers have reported that when the yeast was exposed in a specific acid and high temperature environment, a variety of complex components, such as yeast metabolites and peptone existing in the culture medium, would reduce the external high osmotic pressure and temperature, causing adverse effects on yeast.43 Moreover, an adaptive response mechanism was activated, which promoted the yeast to enhance their defense mechanism for external pressure change, which is known as cross protection.44
Therefore, the yeast exposed to the environment containing a certain amount of lactic and acetic acids, a special substance caused the synthesis to protect yeast strain. More importantly, if the amount of acetic acid is appropriate, it can promote the yeast fermentation.
3.4. Comparison of the pretreatment methods for glycerol production
Glycerol was produced under the osmotic pressure changes and low oxidation–reduction potential.22 In the process of ethanol fermentation, the main function of glycerol is to maintain the balance of NAD+/NADH in yeast cells, and it plays a major role in the beginning of ethanol fermentation.45 In particular, 3-phosphoric acid glycerol dehydrogenase catalyzed the dihydroxy acetone phosphate reduction reaction, which generated 3-phosphoric acid glycerol, and resulted in NADH reduction of NAD+. Then, under the action of 3-glyceride, hydrolysis of glycerol occurred.22 Fig. 5 shows the effects of the different pretreatments on the concentration of glycerol during the SSF process of 5% (w/v) pretreated or raw material (RM) at 38 °C with initial pH = 5.5. As shown in Fig. 5, compared with that in the raw material, the concentration of glycerol remained essentially constant, except for the case of GL–ethanol pretreatment, in which it ranged from 0.489 g L−1 to 0.818 g L−1 throughout the whole SSF. The findings indicated that the reaction with GL–ethanol pretreated material had more inhibitors, and the yeast cells lacked acetaldehyde as a hydrogen acceptor, resulting in an increase in NADH as the final production concentration increased.22 Thus, a high concentration of glycerol was obtained. | ||
| Fig. 5 Comparison of pretreatments for the concentration of glycerol from 5% (w/v) pretreated or raw material (RM) during the SSF process at 38 °C with initial pH = 5.5 for 120 h. | ||
Furthermore, glycerol could be involved in energy metabolism or the synthesis of glycogen and fat. Glycerol not only plays an important role in starting ethanol production, but it can also be used as a metabolic regulation substance for osmotic pressure produced by the high concentration of sugar and ethanol in the fermentation process.46 As reported in previous assays,22 the glucose was over within 24 h, and a number of glycerol molecules was produced in the system to maintain intracellular osmotic pressure. In this study, compared with other pretreatment methods, the lowest amount of glycerol byproduct appeared with the GL–H2O2 pretreatment, suggesting that there existed lower sugar content in the substrate, and this was identical to the lowest yield of ethanol of the GL–H2O2 pretreatment (Fig. 2) during the SSF process. Certainly, glycerol concentration was relatively stable during the SSF process because of the stabilization of ethanol production. The fermentation of pretreated biomass was less affected by glycerol.47
4. Conclusions
In this study, SCB was pretreated by SE, GL–H2O2 and GL–ethanol pretreatments, which was then subjected to SSF. SE pretreatment had a significant impact on the degradation of hemicelluloses and lignin softening, resulting in an increment of glucan content. The results proved that SE pretreatment yielded almost no lactic acid byproduct because a majority of the glucose was consumed for yeast growth and ethanol production. Therefore, the final ethanol yield of 93.86% as the theoretical value at 190 °C for 10 min and 94.33% at 210 °C for 5 min were achieved using a 120 h SSF process. On the other hand, most of the hemicelluloses were removed and more acetyl groups were generated after SE pretreatment, thus it always had the highest amount of acetic acid (1.22 g L−1 at 190 °C for 10 min and 1.38 g L−1 at 210 °C for 5 min, respectively) during the SSF process. GL–ethanol pretreatment broke the steric hindrance of the lignin, achieved the maximum retention of hemicelluloses and obtained the ethanol yield of 80.56%. However, the existence of reactive lignin from GL–ethanol pretreatment led to high byproduct production, especially for the maximum content of glycerol production (0.818 g L−1) during SSF. Deficient lignin removal and some sugar degradation with GL–H2O2 pretreatment led to the lowest ethanol yield of 23.23%, which also may result from the generation of some inhibitors from GL–H2O2 pretreatment, which affect the hydrolysis efficiency and yeast activity in the SSF process. Steam pretreatment with highly digestive cellulose and GL–ethanol pretreatment with more lignin removal and high hemicellulose recovery could be industrialized for sugarcane bagasse bioconversion.Acknowledgements
The authors are grateful for the financial support to this research from the China Ministry of Science and Technology (2014DFG32550) and the Guangxi Key Laboratory of Chemistry and Engineering of Forest Products (GXFC14-06). Authors are grateful to Prof. M. Hakki Alma for providing help in terms of syntax checking.References
- L. Moghaddam, Z. Zhang, R. M. R. M. Wellard, J. P. Bartley, I. M. O'Hara and W. O. S. Doherty, Biomass Bioenergy, 2014, 70, 498–512 CrossRef CAS.
- A. K. Chandel, G. Chandrasekhar, K. Radhika, R. Ravinder and P. Ravindra, Biotechnol. Mol. Biol. Rev., 2011, 6, 8–20 CAS.
- A. Pandey, C. R. Soccol, P. Nigam, V. T. Soccol, L. P. S. Vandenberghe and R. Mohan, Bioresour. Technol., 2000, 74, 69–80 CrossRef CAS.
- L. Canilha, A. K. Chandel, T. S. S. Milessi, F. A. F. Antuns, W. L. C. Freitas, M. G. A. Felipe and S. S. Silva, J. Biomed. Biotechnol., 2012, 2012 DOI:10.1155/2012/989572.
- M. C. D. A. Wanderley, C. Martín, G. J. D. M. Rocha and E. R. Gouveia, Bioresour. Technol., 2013, 128, 448–453 CrossRef PubMed.
- M. M. S. Moretti, D. A. Bocchini-Martins, C. C. C. Nunes, M. A. Villena, O. M. Perrone, R. Silva, M. Boscolo and E. Gomes, App. Energy, 2014, 122, 189–195 CrossRef CAS.
- A. T. W. M. Hendriks and G. Zeeman, Bioresour. Technol., 2008, 100, 10–18 CrossRef PubMed.
- H. L. Yu, Y. Z. You, F. H. Lei, Z. G. Liu, W. M. Zhang and J. X. Jiang, Bioresour. Technol., 2015, 187, 161–166 CrossRef CAS PubMed.
- J. B. Li, P. F. Zhou, H. M. Liu, K. J. Wu, X. L. Kang, Y. X. Gong, W. J. Xiao, J. H. Lin and Z. H. Liu, Ind. Crops Prod., 2014, 62, 446–452 CrossRef CAS.
- A. O. Ayeni, F. K. Hymore, S. N. Mudliar, S. C. Deshmukh, D. B. Satpute, J. A. Omoleye and R. A. Pandey, Fuel, 2013, 106, 187–194 CrossRef CAS.
- G. Banerjee, S. Car, T. Liu, D. L. Williams, S. L. Meza, J. D. Walton and D. B. Hodge, Biotechnol. Bioeng., 2012, 109, 922–931 CrossRef CAS PubMed.
- S. F. Fu, X. S. Shi, F. Wang, X. Z. Yuan and R. B. Guo, RSC Adv., 2015, 5, 63903–63908 RSC.
- S. C. Rabelo, R. Maciel Filho and A. C. Costa, Appl. Biochem. Biotechnol., 2008, 144, 87–100 CrossRef CAS PubMed.
- Y. M. Hong, E. Heikkilä and P. Fardim, Bioresour. Technol., 2013, 150, 36–41 CrossRef PubMed.
- V. Arantes and J. N. Saddler, Biotechnol. Biofuels, 2011, 4, 3 CrossRef CAS PubMed.
- J. Liu, R. Takada, S. Karita, T. Watanabe, Y. Honda and T. Watanabe, Bioresour. Technol., 2010, 101, 9355–9360 CrossRef CAS PubMed.
- X. J. Pan, C. Arato, N. Gilkes, D. Gregg, W. Mabee, K. Pye, Z. Z. Xiao, X. Zhang and J. Saddler, Biotechnol. Bioeng., 2005, 90, 473–481 CrossRef CAS PubMed.
- X. J. Pan, D. Xie, N. Gilkes, D. J. Gregg and J. N. Sadler, Appl. Biochem. Biotechnol., 2005, 121–124, 1069–1079 CrossRef CAS PubMed.
- N. Park, H. Y. Kim, B. W. Koo, H. Yeo and I. Choi, Bioresour. Technol., 2010, 101, 7046–7053 CrossRef CAS PubMed.
- H. L. Yu, T. Yong, X. Yang, L. W. Zhu and J. X. Jiang, Bioresour. Technol., 2013, 147, 29–36 CrossRef CAS PubMed.
- T. Graves, N. V. Narendranath, K. Dawson and R. Power, J. Microbiol. Biotechnol., 2006, 33, 469–474 CAS.
- L. Ji, H. L. Yu, Z. P. Liu, J. X. Jiang and D. F. Sun, Bioresources, 2015, 10, 1162–1173 CAS.
- Z. Zhang, H. H. Wong, P. L. Albertson, M. D. Harrison, W. O. S. Doherty and I. M. O'Hara, Bioresour. Technol., 2015, 192, 367–373 CrossRef CAS PubMed.
- D. A. Abbott and W. M. Ingledew, Biotechnol. Lett., 2004, 26, 1313–1316 CrossRef CAS PubMed.
- C. J. A. D. Souza, D. A. Costa, M. Q. R. B. Rodrigues, A. F. Santos, M. R. Lopes, A. B. P. Abrantes, P. S. Costa, W. B. Silveira, F. M. L. Passos and L. G. Fietto, Bioresour. Technol., 2012, 109, 63–69 CrossRef PubMed.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and D. Crocker, Laboratory Analytical Procedure, 2012 Search PubMed.
- J. M. O. Scurlocka, D. C. Daytonb and B. Hamesb, Biomass Bioenergy, 2000, 19, 229–244 CrossRef.
- Y. Xing, H. Yu, L. W. Zhu and J. X. Jiang, Bioresources, 2013, 8, 5392–5408 CrossRef.
- R. Martín-Sampedro, M. E. Eugenio, J. C. Garcıá, F. Lopez, J. C. Villar and M. J. Diaz, Biomass Bioenergy, 2012, 42, 97–106 CrossRef.
- L. Jiménez, L. Serrano, A. Rodríguez, A. Rodriguez and R. Sánchez, Bioresour. Technol., 2009, 100, 1262–1267 CrossRef PubMed.
- D. Kanungo, S. Omori, R. C. Francis, A. Leavitt and B. Stromberg, J. Wood Chem. Technol., 2011, 31, 267–281 CrossRef CAS.
- G. L. Guo, W. H. Chen, W. H. Chen, L. C. Men and W. S. Hwang, Bioresour. Technol., 2008, 99, 6046–6053 CrossRef CAS PubMed.
- H. L. Yu, X. L. Li, W. M. Zhang, D. F. Sun, J. X. Jiang and Z. G. Liu, Cellulose, 2015, 22, 1675–1686 CrossRef CAS.
- Y. Tang, Z. Q. Su, D. Q. Zhao, X. Qi and J. X. Jiang, Adv. Mater. Res., 2011, 236, 108–111 CrossRef.
- Y. Tang, L. X. Bu, J. He and J. X. Jiang, Eur. Food Res. Technol., 2013, 236, 365–371 CrossRef CAS.
- C. E. Wyman, D. D. Spindler and K. Grohmann, Biomass Bioenergy, 1992, 3, 301–307 CrossRef CAS.
- K. Stenberg, Ph.D. thesis, Dept. of Chemical Engineering 1, Lund University, Sweden, LUTKDH/TKKA-1001/1, 1999.
- S. Marques, J. A. L. Samtos and J. C. Roseiro, Biochem. Eng. J., 2008, 41, 210–216 CrossRef CAS.
- K. C. Thomas, S. H. Hynes and W. M. Ingledew, Appl. Environ. Microbiol., 2002, 68, 1616–1623 CrossRef CAS PubMed.
- N. V. Narendranath, S. H. Hynes, K. C. Thomas and W. M. Ingledew, Appl. Environ. Microbiol., 1997, 63, 4158–4163 CAS.
- H. Lemaresquier, Appl. Microbiol., 1987, 4, 91–94 CrossRef.
- Y. Feng, X. Qi, H. L. Jina, R. C. Sun and J. X. Jiang, Bioresources, 2012, 7, 3755–3766 CrossRef.
- G. Excoffier, A. Peguy, M. Rinaudo and M. Vignon, Potential applications, in International workshop of steam explosion techniques: fundamentals and industrial applications, Milan, Italy, 1991, pp. 83–95 Search PubMed.
- V. Carmelo, R. Santos, C. A. Viegas and I. Sá-Correia, Int. J. Food Microbiol., 1998, 42, 225–230 CrossRef CAS PubMed.
- P. Ribéreau-Gayon, Y. Glories, A. Maujean and D. Dubourdieu, The Chemistry of Wine and Stabilisation and Treatments, John Wiley & Sons, 2nd edn, 2006, vol. 1, DOI:10.1002/0470010398.ch6.
- M. Cantarella, L. Cantarella, A. Gallifuoco, A. Spera and F. Alfani, Biotechnol. Prog., 2004, 20, 200–206 CrossRef CAS PubMed.
- Z. Y. Zhang, H. H. Wong, P. L. Albertson, M. D. Harrison, O. S. D. William and I. M. O'Hara, Bioresources, 2015, 192, 367–373 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |