Effective segregation of cytocompatible chitosan molecules in a silica-surfactant nanostructure formation process†
Abstract
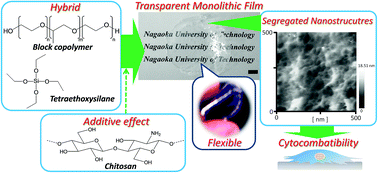
The effective segregation of chitosan (Chi) molecules in a silica-surfactant nanostructure formation process was investigated to find unique self-assembled nanostructures of Chi. The formation process induced the well-defined segregation nanofiber networks to exhibit osteoblast-like cell adhesion and spreading, suggesting the unique Chi segregation nanostructures cytocompatibility.

 Please wait while we load your content...
Please wait while we load your content...