Addressing skin abrasions on artificial turfs with zwitterionic polymer brushes†
Abstract
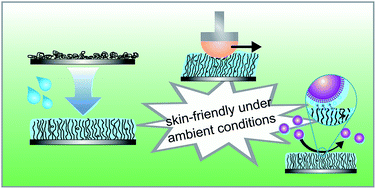
To address the skin-friendliness of synthetic surfaces intended for sports applications, the frictional properties of hydrated zwitterionic polymer brushes are investigated outside of the common aqueous environment where an excess of lubricating water molecules is absent. Photo-grafted poly(sulfobetaine methacrylate) (pSBMA) brushes of various irradiation durations are prepared on polypropylene substrates and the improvement in frictional properties of the pSBMA-modified surfaces against a silicone skin counter-surface is studied. Frictional measurements under both dry and hydrated surface conditions shows that the applied surface modification was capable of forming a stable lubrication layer in the absence of excess water, significantly reducing the coefficient of friction by up to 78.8%. The pSBMA brushes also provide the additional advantage of antifouling – exhibiting resistance towards pathogenic Staphylococcus aureus with almost zero surface colonization for samples irradiated for 1200 s. The low skin-sample friction under ambient conditions and desirable fouling-resistance highlights the potential of pSBMA brushes as a modification strategy for achieving skin-friendly surfaces targeted at reducing the risk of skin abrasions.


 Please wait while we load your content...
Please wait while we load your content...