Properties and unique morphological evolution of dynamically vulcanized bromo-isobutylene-isoprene rubber/polypropylene thermoplastic elastomer
Pengjun Yaoa,
Hanguang Wua,
Nanying Ning*ab,
Liqun Zhangab,
Hongchi Tiana,
Youping Wuab,
Guohua Huc,
Tung W. Chand and
Ming Tian*ab
aKey Laboratory of Beijing City on Preparation and Processing of Novel Polymer Materials, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: tianm@mail.buct.edu.cn; ningny@mail.buct.edu.cn; Fax: +86 10 64433964; Tel: +86 10 64434860
bKey Laboratory of Carbon Fiber and Functional Polymers, Ministry of Education, Beijing University of Chemical Technology, Beijing 100029, China
cLaboratory of Reactions and Process Engineering, University of Lorraine-CNRS, Nancy, France
dDepartment of Materials Science and Engineering, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061, USA
First published on 15th January 2016
Abstract
We studied the microstructure, morphological evolution and the corresponding mechanism, and the properties of bromo-isobutylene-isoprene rubber (BIIR)/polypropylene (PP) thermoplastic vulcanizates (TPVs). Interestingly, a large number of single rubber nanoparticles were observed in the crosslinked BIIR/PP blends, ascribed to the improvement of compatibility between the BIIR and PP with increasing dynamic vulcanization (DV) time, as demonstrated by the increase in interfacial phase thickness and the decrease in the interfacial tension. Most of these single nanoparticles agglomerated as the DV proceeded, leading to the deterioration of the rubber network. Another interesting observation was that the size of rubber agglomerate decreased as the DV proceeded, leading to the strengthening of the rubber network. Importantly, the as-prepared BIIR/PP TPV exhibits good processability, high elasticity and good mechanical property. The relationship between the unique morphology and properties were studied. Our study provides guidance for the preparation of high-performance BIIR/PP TPV for its industrial applications such as medical bottle stoppers.
1 Introduction
Thermoplastic vulcanizates (TPVs) are a special kind of thermoplastic elastomers (TPEs) prepared by dynamic vulcanization (DV), a special reactive polymer blending technique.1–4 During DV, a high-content rubber phase is selectively crosslinked at high temperature and broken up into a dispersed phase in a low-content thermoplastic continuous phase under shear and mixing.4–6 Because of their unique structure,7 TPVs combine the good melt processability and recyclability of thermoplastics and the good elasticity and mechanical property of crosslinked rubbers.8–10 Because of the requirements of environmental protection and resource saving, TPVs as typical “green” polymers have become some of the fastest growing elastomers to replace the unrecyclable petroleum-based thermoset rubbers and have been widely used in industries such as automobile, construction, and electronics.10,11The prerequisite to obtain TPVs is the dispersion of a high content (60–80 wt%) of rubber phase in a small content of continuous plastic phase, usually by using a traditional blending technique.8 In other words, the phase inversion of the rubber phase from the continuous phase to the dispersed phase is the key to obtain TPVs.12–14 The most important properties of TPVs are the elasticity, melt processability and mechanical property, all of which depend on the microstructure of TPV.15 Specifically, the content and crosslinking degree of the rubber phase,16–19 the size of the rubber phase20,21 and the corresponding rubber network structure22,23 and the thickness of the continuous phase15 play key roles in the properties of TPVs. Therefore, the microstructure and the morphological evolution of TPVs during DV have attracted much attention.
Many previous studies reported that the rubber phase in TPVs consists of crosslinked spherical-like particles with diameters of 0.5 to 3.0 μm.24–27 A decrease in the size of the rubber particles results in an increase in the tensile strength and elasticity of the TPV.15 Meanwhile, studies were focused on the morphological evolution of TPV during DV and different mechanisms were proposed. Antunes and Machado28,29 reported that phase inversion was ascribed to the elongation and breaking up of the rubber phase into rubber microparticles dispersed in the plastic matrix in dynamically vulcanized ethylene–propylene–diene terpolymer (EPDM)/polypropylene (PP) TPVs. The mechanism was explained by the rapid increase in the viscosity of the rubber phase due to crosslinking and the shear stress acting on the rubber phase during DV. Goharpey et al.30,31 proposed that the phase inversion was ascribed to the shrinkage and breakdown of the rubber microparticles with a high crosslinking degree during DV because of the shear and the decrease in interfacial adhesion between the two phases. In our previous studies, we proposed a new understanding on the microstructure and the morphological evolution of EPDM/PP TPV. We revealed that the dispersed EPDM microparticles were actually agglomerates of EPDM nanoparticles.32 These nanoparticles were formed by the in situ vulcanization of nanodroplets formed under shear at the early stage of DV. The phase inversion of the EPDM/PP blend during DV was dominated by the formation and agglomeration of rubber nanoparticles,23 and the morphological evolution of the EPDM/PP TPV at the later stage of DV was dominated by the further agglomeration of rubber nanoparticles, leading to the increase in the size of the rubber agglomerates and the thickening of the PP ligament, and the severe deterioration of the rubber network.
Bromo-isobutylene-isoprene rubber (BIIR)/PP TPV has attracted much attention in recent years for its application in medical bottle stoppers. Compared with traditional medical bottle stoppers prepared by thermoset BIIR, the medical bottle stoppers prepared by the BIIR/PP TPV have many advantages. For example, the BIIR/PP TPV stoppers are safer because the continuous PP phase prevents components migrating from rubber phase to liquid medicine and reduces the formation of fragments when needles are penetrated into the bottle stoppers. Meanwhile, the BIIR/PP TPV stoppers can improve production efficiency and save energy because of the easy processability of TPV by using dynamic vulcanization technique. In addition, the BIIR/PP TPV stoppers can save raw materials because of the easy recyclability of TPVs.
In this study, we studied the microstructure, the morphological evolution and the properties of the BIIR/PP TPV. Our goal is twofold. First, we aim to understand the microstructure, properties and the microstructure–property relationship of the BIIR/PP TPV to provide guidance for the preparation of high performance BIIR/PP TPV for its application in medical bottle stoppers. Second, we aim to find out whether the rubber microparticles in the BIIR/PP TPV are formed by the agglomeration of rubber nanoparticles and what the mechanism of morphological evolution in the BIIR/PP TPV is. The morphology of the BIIR/PP blends at various stages of DV was studied. The effect of crosslinking degree on the formation of rubber particles during DV, the mechanism of phase inversion and the variations of the size of rubber phase, the rubber network structure during DV, and properties of the TPV were evaluated.
2 Experimental
2.1 Materials
PP (1956 A) was supplied by Lyondell Basell (Saudi Arabia). BIIR (2030) with a Br content of 1.8 ± 0.2 wt% was supplied by Lanxess (Canada). The BIIR is polymerized by isobutylene (95–98 wt%) and isoprene (1.5–4.5 wt%) and the structural formula of the BIIR is shown in Fig. 1.ZnO and N,N-meta-phenylene bismaleimide (HVA-2) used as crosslinking agents and pentaerythritol tetrakys 3-(3,5-ditert-butyl-4-hydroxyphenyl) propionate (antioxidant 1010) were all commercial products.
2.2 Preparation of the samples
BIIR/PP TPV was prepared in a Haake Rheomix 600 OS internal mixer (Thermo Fisher Scientific, USA) equipped with two counter-rotating rotors. The mass ratio of BIIR/PP was 60/40. The BIIR/PP premix was prepared with the same method as we used in our previous study:32 BIIR and PP were first melt-blended in the Haake Rheomix at 180 °C and then the crosslinking agents were added into the cooled-down premix at room temperature, resulting in a continuous rubber phase. The BIIR/PP blend was dynamically vulcanized in the Haake Rheomix at 180 °C at a rotor speed of 80 rpm. Six samples (designated as samples A–F) with various degrees of vulcanization were selected at various DV times according to the torque–time curve.2.3 Characterization
 | (1) |
Particle size (distribution) was determined based on the AFM images with Image-Pro Plus 4.5 software. At least 50 particles were examined to obtain statistically meaningful results. Moreover, the diameter of an ellipse-like particle is calculated as a half of the sum of the major and minor axes. The number-average diameter (dn), volume-average diameter (dv) and polydispersity index (PDI) were calculated according to the following equations:34
 | (2) |
 | (3) |
 | (4) |
The interparticle distance (IDpoly) was calculated from the relation proposed by Wu35 for polydispersed particles:
 | (5) |
 | (6) |
The surface tension (γ) and its associated dispersion component (γd) and polar component (γp) were calculated according to the Ownes–Wendt–Rabel–Kaelble (OWRK) method (7) and Young's eqn (8):36
 | (7) |
γsg = γsl + γlg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (8) |
| γ = γd + γp | (9) |
The interfacial tension (γ12) between PP and BIIR was obtained from the harmonic mean eqn (10):37
 | (10) |
| ωa = γ1 + γ2 − γ12 | (11) |
3 Results and discussion
3.1 Evolution of crosslinking degree of rubber phase during DV
The crosslinking degree of the rubber phase has a significant effect on the phase morphology of TPV. We first obtained the variation of the crosslinking degree of the rubber phase during DV to study the mechanism of the phase morphology and the morphological evolution of the BIIR/PP TPV. The variation of torque with time during DV is shown in Fig. 2. The torque first decreases to a minimum at point A because of the melting of the blend and then increases to a maximum at point B because of the rapid crosslinking of the rubber phase. Then the torque largely decreases till point C because of the phase inversion and begins to level off at point D. The changes in the crosslinking degree of the rubber phase in the dynamically vulcanized BIIR/PP blends were characterized by the reciprocal volume swell ratio (1/Q) of the six samples collected at points A to F,33 and the results are also shown in Fig. 2. It can be seen that the crosslinking degree of the rubber phase increases rapidly at the early stage of DV (A to C), increases slightly from C to D, and then reaches a plateau at the late stage of DV (D to F), indicating that the crosslinking of the rubber phase mainly occurs at the early stage of DV in the BIIR/PP TPV. The variation of crosslinking degree is similar to that in EPDM/PP blends.23 | ||
| Fig. 2 Variations of torque and reciprocal swell ratio 1/Q with mixing time during DV for BIIR/PP blends. | ||
3.2 Effect of crosslinking degree on formation of rubber nanoparticles
In our previous study, we found that the dispersed rubber microparticles in EPDM/PP TPV are actually the agglomerates of rubber nanoparticles formed by the in situ vulcanization of rubber nanodroplets at the early stage of DV in the EPDM/PP blend.32 The formation of these rubber nanoparticles and their agglomeration play an important role in the morphological evolution of TPV during DV and the rheological property, elasticity and mechanical property. According to the formation mechanism of rubber nanoparticles in EPDM/PP TPV, the rubber nanoparticles could be formed in other kinds of TPVs. Thus, we first studied the effect of the crosslinking degree on the microstructure of the rubber phase in the BIIR/PP blends (samples A to D) during DV by using the PF-AFM, and the results are shown in Fig. 3. As expected, we can observe rubber nanoparticles in all the crosslinked BIIR/PP blends with different crosslinking degrees, similar to those observed in crosslinked EPDM/PP blends. The single rubber nanoparticles and their agglomerates, and the rubber aggregates (formed by the coalescence of rubber nanodroplets with low crosslinking degrees) can be observed in the samples with low crosslinking degree (see Fig. 3(a) and (b)), due to the inhomogeneity of the crosslinking degree of the rubber phase. Some rubber nanodroplets with low crosslinking degrees can still coalesce and form rubber aggregates. With the increase in crosslinking degree, the rubber nanodroplets cannot coalesce but form single rubber nanoparticles and/or agglomerates of nanoparticles (see Fig. 3(c) and (d)). The present study again demonstrates that the dispersed rubber microparticles in TPVs are the agglomerates of rubber nanoparticles, which were formed by the in situ vulcanization of rubber nanodroplets at the early stage of DV. An interesting observation of this study is the formation of a large number of single rubber nanoparticles (see Fig. 3(b)–(d)), as explained in Section 3.4. In addition, this study again demonstrates that the crosslinking degree has a significant effect on the formation of rubber nanoparticles and agglomerates. | ||
| Fig. 3 AFM micrographs of BIIR/PP (60/40) blends with different degrees of crosslinking (the darker regions represent the BIIR phase and the lighter ones represent the PP phase): (a) point A; (b) point B; (c) point C; (d) point D (in Fig. 2). | ||
To further confirm the formation of rubber nanoparticles in the crosslinked BIIR/PP blends, the continuous phase (PP) in sample F was dissolved and washed off with TCB to observe the size and morphology of the crosslinked rubber particles. Fig. 4 shows the AFM image (Fig. 4(a)) of the dissolved sample spin-coated on a clean silicon slice and the corresponding diameter distribution (Fig. 4(b)) of the rubber particles. Interestingly, the diameters of most of the rubber particles are in range of 40 to 100 nm, again demonstrating the formation of rubber nanoparticles in the BIIR/PP TPV. In addition, the rubber microparticles in the BIIR/PP TPV are agglomerates of rubber nanoparticles, similar to those in EPDM/PP TPV.
3.3 Phase morphology and morphological evolution of crosslinked BIIR/PP blends during DV
An interesting observation in this study is that a large number of single rubber nanoparticles are formed in the crosslinked BIIR/PP blends during DV especially in sample B (see Fig. 5(b)) and most of these single rubber nanoparticles agglomerate as the DV proceeds, because of the high surface energy and thermodynamically instability of the nanoparticles.40–42 Another interesting observation is that as the DV proceeds from point C to point F, the size of the rubber agglomerates in the BIIR/PP TPVs decreases, whereas that in the EPDM/PP TPVs increases.23 An explanation for these results will be given in Section 3.4.
The variations of both the rubber nanoparticles and their agglomerates with DV time can significantly affect the rubber network in TPVs. The decrease in the size of rubber agglomerates leads to the strengthening of the rubber network, whereas the decrease in the number of rubber nanoparticles has an adverse effect on the rubber network, which will be further studied by disintegration tests and RPA.
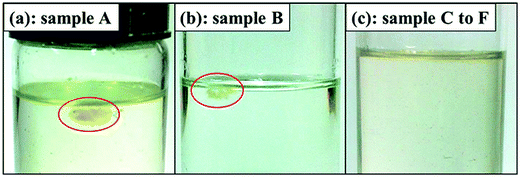
The disintegration tests of samples A to F were carried out to further confirm the phase morphology of the crosslinked BIIR/PP blends because only PP dissolves in TCB, and the photographs of the samples immersed in TCB at 150 °C for 90 h or less are shown in Fig. 6. Fig. 6(a) shows that sample A does not disintegrate even after 90 h, indicating that the rubber phase is the continuous phase. Fig. 6(b) shows that sample B partially disintegrates into small pieces on the surface, an indication of phase inversion. Fig. 6(c) shows that samples C, D, E and F completely disintegrate after 60, 50, 47 and 45 h, respectively, indicating that the phase inversion is complete in these samples; that is, a high-content crosslinked rubber phase is dispersed in a low-content PP continuous phase, consistent with the AFM results. The decrease in disintegration time from samples C to F indicates deterioration of the rubber network as the DV proceeds from C to F. These results again demonstrate the variation of phase structure of the crosslinked BIIR/PP blends during DV, consistent with the AFM results.
| Samples | C | D | E | F |
|---|---|---|---|---|
| dn (nm) | 674 | 507 | 469 | 350 |
| dv (nm) | 896 | 659 | 547 | 398 |
| PDI | 1.33 | 1.30 | 1.17 | 1.14 |
| IDpoly (nm) | 159 | 122 | 104 | 78 |
| Curing time (min) | 2 | 5 | 10 | 15 | 20 |
|---|---|---|---|---|---|
| 1/Q | 0.22 | 0.25 | 0.29 | 0.29 | 0.28 |
| γBIIR (mN m−1) | 35.2 | 34.7 | 30.1 | 29.9 | 29.1 |
| γ12 (mN m−1) | 25.2 | 18.6 | 12.9 | 13.8 | 14.3 |
| ωa (mN m−1) | 58.3 | 64.4 | 65.5 | 64.4 | 63.1 |
3.4 Mechanism of morphological evolution during DV
A schematic illustration of the morphological evolution of the crosslinked BIIR/PP blends during DV is proposed in Fig. 8 to show the formation of rubber nanoparticles and the variations of rubber nanoparticles and nanoparticle agglomerates, which play an important role in the properties of TPVs. The yellow regions represent the PP phase and the blue regions represent the BIIR phase.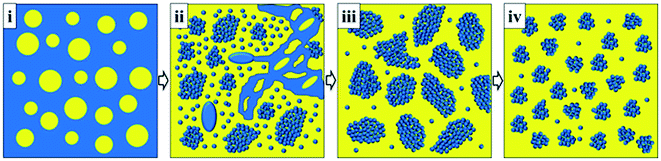 | ||
| Fig. 8 Schematic illustration of morphological evolution of BIIR/PP (60/40) TPV during DV (the yellow regions represent PP phase and the blue regions represent the BIIR phase). | ||
In the uncrosslinked BIIR/PP blend, the PP phase (40 wt%) is dispersed in the uncrosslinked BIIR continuous phase (60 wt%) (see Fig. 8(i)). At the early stage of DV, the breakup of the BIIR phase under rotational shear can result in the formation of a large number of BIIR nanodroplets, as demonstrated in our previous study.23,32 Some of the nanodroplets at low crosslinking degrees can still coalesce to form rubber aggregates, whereas some of these nanodroplets at high crosslinking degrees transform into rubber nanoparticles and/or nanoparticle agglomerates. As a result, large numbers of rubber nanoparticles, nanoparticles agglomerates and rubber aggregates exist simultaneously at this stage, and the blend exhibits a co-continuous structure (see Fig. 8(ii)). As the DV proceeds, the BIIR nanodroplets cannot coalescence but disperse in the PP matrix as single rubber nanoparticles and/or nanoparticle agglomerates because of the increase in crosslinking degree, resulting in the phase inversion (see Fig. 8(iii)). With further increases in DV time, many of the single rubber nanoparticles agglomerate, resulting in the decrease in the number of single nanoparticles, whereas the size of the rubber agglomerates decreases, resulting in the increase in the number of rubber agglomerates (see Fig. 8(iv)). The formation of many single rubber nanoparticles and the decrease in the size of the rubber nanoparticle agglomerates in the BIIR/PP blends as the DV proceeds is a new discovery in the morphological evolution of TPV, quite different from the morphological evolution in the EPDM/PP TPVs.23
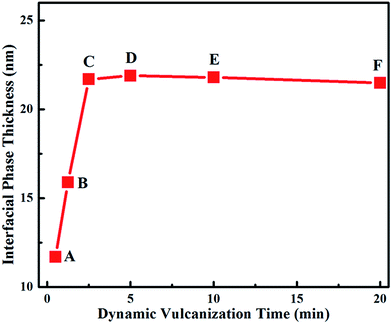
To understand the corresponding mechanism, the variation of the interfacial phase thickness between the BIIR and PP phases and the simulated evolution of the surface tension of the BIIR phase and interfacial tension between the BIIR and PP phases were studied. The increase in compatibility between the BIIR and PP can be indicated by the variation of interfacial phase thickness during DV measured on the AFM images. It can be seen in Fig. 9 that the interfacial phase thickness between the BIIR and PP phase increases rapidly as DV proceeds from A to C, demonstrating the improvement of the compatibility because of the reaction between PP and BIIR under high temperature and high shear during DV. The improvement of compatibility between BIIR and PP facilitates the formation of single rubber nanoparticles at the early stage of DV (A to C).
The interfacial tensions between PP and statically vulcanized BIIRs with different curing times were measured and calculated at room temperature to simulate the variation of interfacial tension during DV because the surface tension (γ) of a polymer varies linearly with temperature.30 Moreover, the statically vulcanized BIIR samples with curing time of 2 min, 5 min and 10 min can be regarded as samples A, B and C, respectively, because of the similar crosslinking degrees measured by the reciprocal volume swell ratio (1/Q), and the result is shown in Table 2. The interfacial tension (γ12) between the BIIR and PP phases decreases with the increase in crosslinking time (2 min to 10 min) whereas the adhesion work (ωa) increases, again suggesting the improvement in compatibility between the BIIR and PP phases to facilitate the formation of single rubber nanoparticles.
The cohesive energy of rubber agglomerates plays an important role in their formation at the late stage of DV from C to F. A lower cohesive energy can lead to smaller rubber agglomerates. According to Lee,43 the critical surface tension is related to the cohesive energy as follows:
| γc0.43 = KδφV0.24 | (12) |
3.5 Properties of BIIR/PP TPV
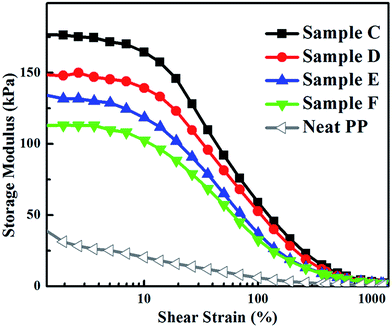
The phase morphology and morphological evolution have significant effects on the properties of the BIIR/PP TPV. Fig. 10–12 show the rheological property, elasticity and tensile properties, respectively, of the BIIR/PP TPV as the DV proceeds from C to F. | ||
| Fig. 10 Storage modulus (G′) and complex viscosity (η*) versus angular frequency (ω) from samples C to F. | ||
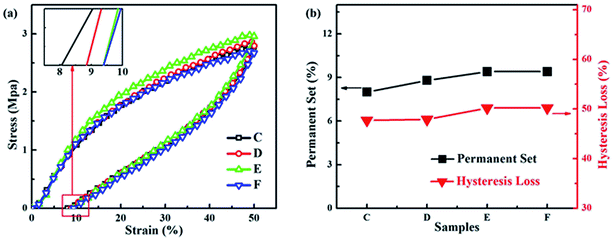 | ||
| Fig. 11 (a) Tensile recovery curves and (b) variations of permanent set and hysteresis loss from samples C to F. | ||
 | ||
| Fig. 12 (a) Stress–strain curves and (b) variations of tensile stress and elongation at break from samples C to F. | ||
Rheological property, dominated by the continuous plastic matrix, plays an important role in the melt processability, the recyclability and the production efficiency of TPVs. The rheological property during DV was studied by using RPA and the results of samples C to F are shown in Fig. 10. Low storage modulus (G′) and complex viscosity (η*) represents easy processability, easy recyclability, and high production efficiency of TPVs. It can be seen that both η* and G′ slightly decrease from C to F at a given frequency, suggesting that the melt processability of the samples improves with the increase in DV time. The improvement in processability is ascribed to the slight deterioration of the rubber network as the DV proceeds from C to F, as demonstrated by the disintegration tests and RPA results (see Section 3.3). The slight deterioration of the rubber network is ascribed to the combined effect of the deterioration of the rubber network caused by the significant decrease in the number of single rubber nanoparticles and the strengthening of the rubber network by the increase in the number of rubber nanoparticle agglomerates (decrease in the thickness of the PP matrix). More importantly, all these TPV samples from C to F show easy processability, facilitating the industrial applications of the BIIR/PP TPV. In addition, compared with traditional thermoset BIIR, our BIIR/PP TPV can save raw materials by recycling the leftover materials during production because of the good melt processability of TPVs.
Elasticity caused by the high content and high crosslinking degree of the BIIR phase in TPV, has significant effects on the hardness and the dynamic fatigue-resistance of TPV products. The tensile recovery tests of samples C to F were carried out to study the elasticity of the samples. Hysteresis loss and permanent set summarized according to the tensile recovery curves are two important parameters to represent the elasticity of elastomers. Low permanent set and hysteresis loss represents high elasticity.44,45 It can be seen from Fig. 11 that both the permanent sets and the hysteresis losses of samples C to F increase, indicating a slight decrease in the elasticity of the BIIR/PP TPV with the DV time. As the content and the crosslinking degree of the rubber are the same from samples C to F, the slight decrease in elasticity is attributed to the slight deterioration of the rubber network caused by the combined effect of the significant decrease in the number of single rubber nanoparticles and the significant increase in the number of rubber nanoparticle agglomerates (decrease in the thickness of the PP matrix). In addition, our BIIR/PP TPV exhibit high elasticity, as demonstrated by the permanent set (8% to 10%) of the BIIR/PP TPV samples according to ASTM D1566-07a.
Fig. 12 shows the stress–strain curves of samples C to F and the variations of the tensile stress and the elongation at break as the DV proceeds from C to F. It can be seen that both the tensile strength and the elongation at break are almost the same for all the samples because of the combined effect of the significant decrease in the number of single rubber nanoparticles, which decreases the tensile properties, and the significant increase in the number of rubber nanoparticle agglomerates, which increases the tensile properties. In addition, the tensile strength is larger than 12 MPa and the elongation at break is larger than 300% for samples C to F, indicating the good mechanical strength and good toughness of the BIIR/PP TPV.
Therefore, we have successfully prepared BIIR/PP TPV with good elasticity, easy processability, good mechanical property and easy recyclability. Our BIIR/PP TPV has three advantages over traditional thermoset BIIR. First, the BIIR/PP TPV has good processability and recyclability and thus can save cost and protect the environment. Second, the BIIR/PP TPV is more safety when used as medical bottle stoppers because there is no fragment when penetrated into the bottle stoppers. Third, the BIIR/PP TPV is of low toxicity because of few additives and continuous PP phase.
4 Conclusions
The microstructure, morphological evolution, and the properties of the BIIR/PP TPV were studied. The corresponding mechanism for the formation and evolution of the microstructure was proposed based on the crosslinking degree of the rubber phase, and the compatibility and interfacial tension between BIIR and PP. The results indicate that the dispersed rubber microparticles in the BIIR/PP TPV are actually agglomerates of rubber nanoparticles and the phase inversion of the BIIR/PP blend during DV is dominated by the formation and agglomeration of rubber nanoparticles, consistent with those observed in EPDM/PP TPVs. Interestingly, a large number of single rubber nanoparticles are formed in the crosslinked BIIR/PP blends because of the improvement in compatibility between BIIR and PP with the increase in crosslinking degree, as demonstrated by the increase in interfacial phase thickness and adhesion work as well as the decrease in interfacial tension. As the DV proceeds, most of the single nanoparticles agglomerate because of the high surface energy of the nanoparticles and their thermodynamical instability, leading to the deterioration of the rubber network. In addition, the size of the rubber agglomerates and the thickness of the PP matrix decrease as DV further proceeds because of the decrease in surface tension of BIIR and the decrease in cohesive energy of the rubber agglomerates, leading to the strengthening of the rubber network. As a result, a slight variation of the rubber network was obtained, leading to the slight changes in elasticity, processability and mechanical properties of the TPVs as the DV proceeds. Importantly, the as-prepared BIIR/PP TPV exhibits good processability, high elasticity and good mechanical property, facilitating the application of the BIIR/PP TPV in medical bottle stoppers.Acknowledgements
We gratefully acknowledge the National Science Fund for Distinguished Young Scholars of China (Grant No. 51525301), the National Basic Research Program of China (Grant No. 2011CB606003), the National Natural Science Foundation of China (Grant No. 51221002) and the Doctoral Science Research Foundation of the Education Ministry of China (Grant No. 20130010110005) for financial supports. We are also thankful to Liyu Dong Wu for experimental help.References
- Y. Tang, K. Lu, X. Cao and Y. Li, Ind. Eng. Chem. Res., 2013, 52, 12613–12621 CrossRef CAS
.
- A. V. Machado and M. van Duin, Polymer, 2005, 46, 6575–6586 CrossRef CAS
.
- Y. Chen, C. Xu, X. Liang and L. Cao, J. Phys. Chem. B, 2013, 117, 10619–10628 CrossRef CAS PubMed
.
- C. F. Antunes, A. V. Machado and M. van Duin, Rubber Chem. Technol., 2009, 82, 492–505 CrossRef CAS
.
- H. Huang, J. L. Yang, X. Liu and Y. X. Zhang, Eur. Polym. J., 2002, 38, 857–861 CrossRef CAS
.
- J. Oderkerk and G. Groeninckx, Polymer, 2002, 43, 2219–2228 CrossRef CAS
.
- R. l'Abee, H. Goossens, M. van Duin and A. Spoelstra, Eur. Polym. J., 2009, 45, 503–514 CrossRef
.
- C. F. Antunes, M. van Duin and A. V. Machado, Polym. Test., 2011, 30, 907–915 CrossRef CAS
.
- Z. Li and M. Kontopoulou, Polym. Eng. Sci., 2009, 49, 34–43 CAS
.
- M. van Duin, Macromol. Symp., 2006, 233, 11–16 CrossRef CAS
.
- K. Lu, M. van Duin, J. Loos and G. de With, Polymer, 2012, 53, 4171–4177 CrossRef CAS
.
- C. F. Antunes, A. V. Machado and M. Van Duin, Eur. Polym. J., 2011, 47, 1447–1459 CrossRef CAS
.
- G. Jordhamo, J. Manson and L. Sperling, Polym. Eng. Sci., 1986, 26, 517–524 CAS
.
- S. Steinmann, W. Gronski and C. Friedrich, Polymer, 2001, 42, 6619–6629 CrossRef CAS
.
- R. l'Abee, M. van Duin, A. Spoelstra and J. Goossens, Soft Matter, 2010, 6, 1758–1768 RSC
.
- R. R. Babu, N. K. Singha and K. Naskar, eXPRESS Polym. Lett., 2010, 4, 197–209 CrossRef CAS
.
- M. D. Ellul, A. H. Tsou and W. Hu, Polymer, 2004, 45, 3351–3358 CrossRef CAS
.
- G. Martin, C. Barres, P. Sonntag, N. Garois and P. Cassagnau, Eur. Polym. J., 2009, 45, 3255–3266 CrossRef
.
- P. Yao, M. Tian, L. Zhang, H. Tian, Y. Wu and N. Ning, J. Appl. Polym. Sci., 2014, 131, 9255–9261 Search PubMed
.
- R. C. Willemse, E. J. J. Ramaker, J. van Dam and A. Posthuma de Boer, Polymer, 1999, 40, 6651–6659 CrossRef CAS
.
- K. Premphet and W. Paecharoenchai, J. Appl. Polym. Sci., 2002, 85, 2412–2418 CrossRef CAS
.
- L. Wang, N. Ning, L. Zhang, Y. Lu, M. Tian and T. Chan, Composites, Part A, 2013, 47, 135–142 CrossRef CAS
.
- H. Wu, M. Tian, L. Zhang, H. Tian, Y. Wu, N. Ning and T. W. Chan, ACS Sustainable Chem. Eng., 2015, 3, 26–32 CrossRef CAS
.
- P. A. Bhadane, N. Virgilio, B. D. Favis, M. F. Champagne, M. A. Huneault and F. Tofan, AIChE J., 2006, 52, 3411–3420 CrossRef CAS
.
- A. Y. Coran and R. Patel, Rubber Chem. Technol., 1980, 53, 141–150 CrossRef CAS
.
- H. Huang, T. Ikehara and T. Nishi, J. Appl. Polym. Sci., 2003, 90, 1242–1248 CrossRef CAS
.
- A. Mousa, Int. J. Polym. Mater., 2005, 54, 619–631 CrossRef CAS
.
- C. F. Antunes, M. van Duin and A. V. Machado, Mater. Chem. Phys., 2012, 133, 410–418 CrossRef CAS
.
- A. V. Machado, C. F. Antunes and M. van Duin, Novel Trends in Rheology IV, 2011, vol. 1375, pp. 240–247 Search PubMed
.
- F. Goharpey, A. Katbab and H. Nazockdast, J. Appl. Polym. Sci., 2001, 81, 2531–2544 CrossRef CAS
.
- F. Goharpey, A. Katbab and H. Nazockdast, Rubber Chem. Technol., 2003, 76, 239–252 CrossRef CAS
.
- H. Wu, M. Tian, N. Ning, L. Zhang, Y. Wu and H. Tian, Soft Matter, 2014, 10, 1816–1822 RSC
.
- N. Vennemann, K. Bökamp and D. Bröker, Macromol. Symp., 2006, 245, 641–650 CrossRef
.
- I. Aravind, K. H. Ahn, C. Ranganathaiah and S. Thomas, Ind. Eng. Chem. Res., 2009, 48, 9942–9951 CrossRef CAS
.
- S. Wu, J. Appl. Polym. Sci., 1988, 35, 549–561 CrossRef CAS
.
- D. K. Owens and R. Wendt, J. Appl. Polym. Sci., 1969, 13, 1741–1747 CrossRef CAS
.
- S. Wu, Polymer interface and adhesion, Marcel Dekker, New York, 1982 Search PubMed
.
- A. Y. Coran and R. Patel, Rubber Chem. Technol., 1983, 56, 210–225 CrossRef CAS
.
- S. Chattopadhyay, T. Chaki and A. K. Bhowmick, J. Appl. Polym. Sci., 2001, 79, 1877–1889 CrossRef CAS
.
- M. E. Mackay, A. Tuteja, P. M. Duxbury, C. J. Hawker, B. Van Horn, Z. Guan, G. Chen and R. S. Krishnan, Science, 2006, 311, 1740–1743 CrossRef CAS PubMed
.
- K. K. Nanda, A. Maisels, F. E. Kruis, H. Fissan and S. Stappert, Phys. Rev. Lett., 2003, 91, 1–60 CrossRef PubMed
.
- M. Wang, Rubber Chem. Technol., 1999, 72, 430–448 CrossRef CAS
.
- L. Lee, J. Polym. Sci., Polym. Phys. Ed., 1967, 5, 1103–1118 Search PubMed
.
- J. Oderkerk, G. Groeninckx and M. Soliman, Macromolecules, 2002, 35, 3946–3954 CrossRef CAS
.
- J. Oderkerk, G. de Schaetzen, B. Goderis, L. Hellemans and G. Groeninckx, Macromolecules, 2002, 35, 6623–6629 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2016 |