Modification of mechanical properties of vertical graphene sheets via fluorination†
Keivan Davami*ab,
Yijie Jianga,
Chen Lina,
John Cortesa,
Jeremy T. Robinsonc,
Kevin T. Turnera and
Igor Bargatin*a
aDepartment of Mechanical Engineering and Applied Mechanics, University of Pennsylvania, Philadelphia, PA 19104, USA. E-mail: kdavami@seas.upenn.edu; bargatin@seas.upenn.edu
bDepartment of Mechanical Engineering, Widener University, One University Place, Chester, PA 19013, USA
cNaval Research Laboratory, Washington, D.C. 20375, USA
First published on 21st January 2016
Abstract
We report systematically tuning the mechanical properties of vertical graphene (VG) sheets through fluorination. VG sheets were synthesized using a radio-frequency plasma-enhanced chemical vapor deposition (RF-PECVD) technique and were functionalized through exposure to xenon difluoride (XeF2) gas. An atomic force microscopy technique, PeakForce Quantitative Nanomechanical Mapping (QNM®), was used to measure the mechanical properties of the VG sheets. We show that fluorination can significantly enhance the reduced modulus of surfaces comprised of VG sheets. Samples with only ∼3.5% fluorine had a reduced modulus approximately eight times higher than unfunctionalized VG sheets, which is attributed to sp2 to sp3 conversion and a change of the C–C bond length after functionalization. Fluorination also decreased the energy dissipation of the VG sheets and reduced their adhesion to the AFM tip. This method represents a unique approach towards modification of the mechanical properties of nanostructures without a significant increase in weight or change of the VG sheet morphology.
1. Introduction
Manipulating the properties of sp2 carbon-based nanostructures (e.g., carbon nanotubes and graphene) in order to tailor their characteristics for different applications is an important technique in nanoengineering. There are many reports on tuning the mechanical, electrical, and thermal properties of various carbon-based nanostructures, all with the goal of extending their potential application space.1–4 These studies not only open new avenues for creating novel devices, but also lay the groundwork for developing a thorough understanding of physical phenomena and interactions between nanostructures that might lead to new insight into low dimensional systems.Although modification of the electrical and thermal conductivities can be done readily without changing the morphologies of the nanostructures through a wide range of techniques (e.g. annealing or doping), tuning the mechanical properties is not as straightforward. In the absence of direct chemical/stoichiometric modification, techniques to tune mechanical properties usually involve coating the surface of the nanostructures with a continuous or discontinuous layer of another material.5,6 Such coating approaches alter the properties of the nanostructures, but are also accompanied by a dramatic alteration in their morphologies, surface-to-volume ratios, and their weight. Also, creating an additional interface between the nanostructures and the deposited layer can negatively affect heat and charge transport.5
Enhancing the Young's modulus of graphene through the introduction of defects with a controlled density via bombardment of pristine graphene samples with Ar+ ions has been recently reported.7 Specifically, the in-plane Young's modulus of graphene almost doubled when the defect content increased to ∼0.2% but degraded when the defect density was increased further.7 The enhancement of the Young's modulus is attributed to the out-of-plane fluctuations being reduced by defects.8 The limitations of this method include the relatively narrow range of increase in the 2D elastic modulus (305 N m−1 for pristine compared to 550 N m−1 for modified), requirement of complex facilities to accurately induce defects with required density, and degradation of the strength. Thus, new techniques to adjust the mechanical properties of nanostructures without changing their morphologies or adding extra material are highly desired, particularly since many of the attractive properties of nanostructures depend on their lightweight and delicate ultrathin atomic-scale morphologies.
Functionalization through the introduction of chemical groups to the surface of an sp2-bonded carbon sheet and creating an sp3-hybridized carbon film remains a powerful tool for developing graphene-based applications.9–11 The addition of fluorine to graphene's basal plane results in the creation of graphene fluoride, a two-dimensional sheet of sp3-hybridized carbon.12 Significant changes in the optical and electronic properties of graphene after functionalization with fluorine have been reported before.12,13 Fluorination also affects other properties of the graphene sheets such as surface roughness, hydrophilicity, and hydrophobicity.14
Here we show how fluorination can be used to tune the mechanical properties of vertical graphene (VG) sheets, also known as carbon nanowalls.15 These nanostructures are flakes of carbon that are composed of 1 to 20 layers of graphene and grow catalyst-free vertically through chemical vapor deposition methods on top of various substrates.15 Multiple applications, such as nanocomposites and light absorbers have been proposed for carbon nanowalls, and mechanical properties play a critical role in these applications.16,17 According to the results below, adding only a few atomic percent of fluorine to these nanostructures dramatically changes their mechanical properties. This study thus demonstrates a new, simple method that can be used to tune the mechanical properties of VG sheets inexpensively and at large scale.
2. Materials and methods
An RF-PECVD method was used for the growth of the VG sheets on copper substrates. The details of the growth process were reported previously.15 The copper substrates were cut into small pieces with approximate dimensions of 1 × 1 cm2. The samples were cleaned with acetone and IPA and were rinsed with DI water. Subsequently, the samples were dried with N2. Initially and in between each growth process, the RF-PECVD chamber was cleaned with oxygen plasma for a period of 90 min while the plasma power and sample stage temperature were maintained at 900 W and 750 °C respectively. This process removes amorphous carbon that usually forms inside the chamber while depositing carbon-based materials. The samples were placed on a stage with resistive heating capability of a homemade RF-PECVD inside the chamber. The temperature of the stage was controlled through an external controller. The pressure of the chamber was reduced to 1 Torr and the stage temperature was raised up to 680 °C at a rate of 22 °C min−1. Hydrogen and methane with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio were inserted into the chamber to ignite the plasma while the RF power was set at 900 W. After 40 min of deposition, the process was stopped and the samples were cooled down in vacuum to room temperature to avoid their oxidation.
1 ratio were inserted into the chamber to ignite the plasma while the RF power was set at 900 W. After 40 min of deposition, the process was stopped and the samples were cooled down in vacuum to room temperature to avoid their oxidation.
In order to functionalize the samples, they were placed in the chamber of an Xactix XeF2 isotropic etcher and were exposed to XeF2 gas for varying numbers of etch cycles, similar to the method in ref. 18. XeF2 does not attack the copper substrate.19 The cycle time (30 s), delay between two sequential cycles (2 s), and XeF2 pressure (2 Torr) were the same for all samples. Different samples were exposed to XeF2 for 50, 100, 200, 300, 400, and 500 cycles to produce VG sheets with different fluorine content.
A scanning electron microscope (SEM - JEOL 7500 HRSEM) operating at 5.00 kV was used for imaging the morphology of the VG sheets. In order to study the structure of the VG sheets a JEOL JEM 2100 transmission electron microscope (TEM) operating at 200 kV was used. TEM samples were prepared by scratching the VG substrate by copper grids slightly, and then they were studied in the TEM. The details of the TEM studies were reported previously in our other report.15 Raman spectroscopy analysis was done using an NT-MDT NTEGRA system with an excitation wavelength of 532 nm, an exposure time of 10 seconds, and a laser power of 0.2 mW. A Bruker ICON AFM was used for the mechanical characterization. The samples were glued to aluminum disks and the disks were mounted on a vacuum stage. The tip radius was measured using SEM. To calibrate the AFM probe, the detector sensitivity was first measured by pushing the AFM probe into contact with a hard surface, and then the cantilever stiffness was determined using the thermal tune method.
3. Results and discussion
When VG sheets are exposed to XeF2, their surface layers become fluorinated.12,20 The fluorinated graphene has a non-stoichiometric composition, and fluorine atoms are most likely first adsorbed at lower energy binding sites, such as vacancy defects (see Raman spectra, Fig. S1†). Since VG sheets are vertically oriented and both surfaces are exposed to XeF2, we expect that single-layer VG sheets will reach 100% fluorine coverage.12 However, under the current processing conditions the majority of VG sheets have more than one layer, and we expect fluorine adsorption on the outmost graphene layers only, without intercalation between the layers.Fig. 1(a)–(d) show scanning SEM images of the VG sheets before and after functionalization, respectively. No visible change in the morphology of the VG sheets is observed after XeF2 treatment. We attribute the reduced sharpness in the SEM images of the functionalized VG sheets (Fig. 1(c) and (d)) compared to the pristine sheets (Fig. 1(a) and (b)) to the reduction in the conductivity after fluorination, which leads to more charging during SEM imaging.12 A cross section image of the pristine VG sheets on a copper substrate is presented in Fig. S2(a) in ESI.† A high resolution transmission electron microscope (TEM) image of the sheets is shown in Fig. S2(b).† The microscopy characterization results were reported comprehensively in our previous work in ref. 15.
 | ||
| Fig. 1 SEM pictures of (a and b) pristine and (c and d) functionalized samples (400 cycles). Scale bars in (a and c) and (b and d) are 100 nm and 10 nm, respectively. | ||
We measured the fluorine content at four different locations (∼1 μm2 each) on each fluorinated sample using an energy-dispersive X-ray spectroscopy (EDS) technique in the SEM. An acceleration voltage of 2.0 kV was used to prevent incident electrons from reaching the substrate (VG film thickness is ∼480 nm). Fig. 2 shows the relationship between the fluorine content of sample and the number of the cycles of exposure to XeF2. Similar to other reports12 the fluorine content increases with the number of the fluorination cycles, up to 200 cycles here (6000 seconds of total exposure) and then saturates at an average EDS measured concentration of approximately 3.5%. The atomic percentages of carbon and fluorine for different samples are shown in Table S1 in ESI.† It should be noted that the technique used to add fluorine in these experiments, XeF2 gas exposure at room temperature, is effective at adding fluorine to the surface of the VG sheets. As shown in ref. 12, XeF2 treatment does not add additional fluorine to the inner layers of graphene multilayers under these conditions. At ∼3.5% a saturation level is achieved and longer exposure to XeF2 does not increase the fluorine content.
 | ||
| Fig. 2 Fluorine content versus cycles of functionalization. Each data point in the plot above is the average value of four measurements at different locations of each sample using an EDS technique. | ||
The Raman spectra did not change dramatically with fluorination (Fig. S1†), largely impart due to the numerous edges in the VG sheets that contribute to the D peak (1350 cm−1) intensity. In these experiments fluorine is likely only attaching on the surface of the sheets while most characterization techniques, including Raman and EDS, average over all carbon atoms in the systems, making that the surface contribution less important.
An AFM-based method known as PeakForce QNM®21,22 was used to characterize the mechanical properties of the VG sheets as function of fluorination. The QNM is a tapping mode approach to characterize the mechanical properties of surfaces. The method allows for control of the maximum force during the test and the tapping mode scanning minimizes the lateral forces that are applied to the sample. These characteristics both reduce the potential for sample damage from the measurement. All measurements in this report were performed using a maximum load of 100 nN. A special development probe from Nanosensors™ (type: SD-sphere-FM-L) with a tip diameter of ∼4.5 μm (Fig. S3 in ESI†) and a stiffness of ∼2.5 N m−1 was used for the measurements. The tip was much larger than a typical AFM tip that is usually used for imaging23,24 and was intentionally chosen in order to measure the average mechanical properties of the VG films, which have a complex surface geometry at small length scales.
The QNM measurements yield force–displacement indentation curves from many locations on the sample. The effective reduced modulus (Er = E/(1 − ν2)), where E is the Young's modulus and ν is the Poisson's ratio was calculated from the unloading segment of the force–displacement curves using a contact mechanics model assuming Derjaguin–Muller–Toporov (DMT) adhesion.25 Furthermore, adhesive pull-off forces were also extracted from the unloading curves. Fig. 3(a) shows the effective reduced moduli of samples with different degrees of XeF2 exposure. Due to the unique structure of the surfaces, these reduced modulus values represent the effective elastic properties of the surfaces and depend on the properties and geometry of the VG structures. The addition of approximately 3% fluorine increases the effective reduced modulus by approximately a factor of 8. This is attributed to differences in the C–C and C–F bond lengths,26,27 the interlayer distance between the layers of carbon in the multi-layer VG sheets, and a change in the ratio of sp2 to sp3-hybridized carbon.10 Notably, a similar enhancement in the mechanical properties of single-layer fluorinated horizontal graphene has been reported before.28,29 Specifically, the out-of-plane normal stiffness and bending stiffness of graphene were calculated to increase by a factor of 4 after fluorination in ref. 28. Fig. 3(b) shows the adhesion force between the tip and the VG sheets before and after functionalization. Fluorination reduces the adhesion force between the AFM tip and VG sheets. This is likely due to the protruding C–F bonds reducing the van der Waals force between the tip and the graphene.28
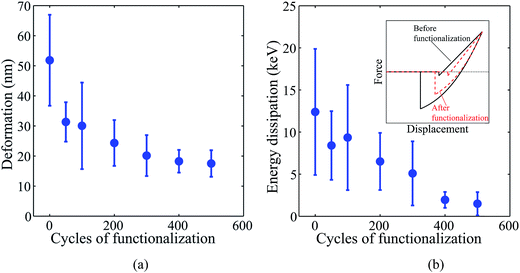
Fig. 4(a) compares the deformation of pristine and fluorinated VG sheets under controlled loading. The deformation was calculated from the approach part of the force–displacement curve by taking into account the cantilever deflection. Fluorinated samples deform much less under the load of 100 nN; with only fifty cycles of XeF2 resulting in a decrease in deflection of over 40% relative to pristine samples. One explanation for this decrease is the overall increase in the reduced modulus of VG sheets (Fig. 3(a)). However, the deformation is not simply inversely proportional to the reduced modulus as for large displacements the response is nonlinear. The collected data, however, does clearly show a trend that can be explained by functionalization of defects and the partial phase change (sp2 to sp3) in carbon due to fluorination.10
Energy dissipation, calculated as the area enclosed between the loading and unloading curves, decreases with increasing fluorine content (Fig. 4(b)). The average energy dissipation for as-grown VG sheets samples is about 12.8 keV, while that for maximum fluorine treated (500 cycle XeF2) samples is more than 7 times lower at approximately 1.5 keV. Two main factors likely contribute to this decrease. First, fluorinated VG sheets show smaller deformation which reduces the friction loss and as a result, reduces energy dissipation. Second, as can be seen from Fig. 3(b), the average adhesion force generally decreases with the number of functionalization cycles, which also contributes to lower energy dissipation compared to pure VG sheets (see the inset in Fig. 4(b)). Representative images of QNM output are shown in Fig. S4.† Measurements were also performed with a second probe to verify the results (Table S3†).
4. Conclusion
Coating techniques previously used for tuning the mechanical properties of carbon-based nanostructures dramatically increase their weight and change their morphology, limiting possible applications. Here, we directly modify the mechanical properties of VG sheets through functionalization with fluorine. When VG sheets are exposed to XeF2 gas, fluorine atoms bond with carbon atoms until fluorine concentration reaches the saturation level of ∼3.5 atomic percent. The reduced modulus of the VG sheets increases with increasing fluorine content, while their deformation and energy dissipation both decrease. These changes in mechanical properties are attributed to the sp2 to sp3 conversion and the change of the C–C bond length after functionalization.Acknowledgements
This work was supported by a seed grant of the Center of Excellence for Materials Research and Innovation (CEMRI) at the University of Pennsylvania, NSF grant DMR-1120901 and the School of Engineering and Applied Science at the University of Pennsylvania.References
- H. Tanaka, R. Arima, M. Fukumori, D. Tanaka, R. Negishi, Y. Kobayashi, S. Kasai and T. K. Yamada, Method for Controlling Electrical Properties of Single-Layer Graphene Nanoribbons via Adsorbed Planar Molecular Nanoparticles, Sci. Rep., 2015, 5, 12341 CrossRef PubMed.
- J.-K. Lee, S. Yamazaki, H. Yun, J. Park, G. P. Kennedy, G.-T. Kim, O. Pietzsch, R. Wiesendanger, S. Lee, S. Hong, U. Dettlaff-Weglikowska and S. Roth, Modification of Electrical Properties of Graphene by Substrate-Induced Nanomodulation, Nano Lett., 2013, 13(8), 3494–3500 CrossRef CAS PubMed.
- J. Zhang, P. Loya, C. Peng, V. Khabashesku and J. Lou, Quantitative In Situ Mechanical Characterization of the Effects of Chemical Functionalization on Individual Carbon Nanofibers, Adv. Funct. Mater., 2012, 22(19), 4070–4077 CrossRef CAS.
- S. Chen, Q. Wu, C. Mishra, J. Kang, H. Zhang, K. Cho, W. Cai and A. A. Balandin, Thermal conductivity of isotopically modified graphene, Nat. Mater., 2012, 11(3), 203–207 CrossRef CAS PubMed.
- A. Kumar, M. R. Maschmann, S. L. Hodson, J. Baur and T. S. Fisher, Carbon nanotube arrays decorated with multi-layer graphene-nanopetals enhance mechanical strength and durability, Carbon, 2015, 84, 236–245 CrossRef CAS.
- A. Brieland-Shoultz, S. Tawfick, S. J. Park, M. Bedewy, M. R. Maschmann, J. W. Baur and A. J. Hart, Scaling the Stiffness, Strength, and Toughness of Ceramic-Coated Nanotube Foams into the Structural Regime, Adv. Funct. Mater., 2014, 24(36), 5728–5735 CrossRef CAS.
- G. Lopez-Polin, C. Gomez-Navarro, V. Parente, F. Guinea, M. I. Katsnelson and F. Perez-Murano, Increasing the elastic modulus of graphene by controlled defect creation, Nat. Phys., 2015, 11(1), 26–31 CrossRef CAS.
- M. I. Katsnelson and A. Fasolino, Graphene as a Prototype Crystalline Membrane, Acc. Chem. Res., 2013, 46(1), 97–105 CrossRef CAS PubMed.
- Q. Peng, A. K. Dearden, X.-J. Chen, C. Huang, X. Wen and S. De, Peculiar pressure effect on Poisson ratio of graphone as a strain damper, Nanoscale, 2015, 7, 9975–9979 RSC.
- A. G. Kvashnin, L. A. Chernozatonskii, B. I. Yakobson and P. B. Sorokin, Phase Diagram of Quasi-Two-Dimensional Carbon, From Graphene to Diamond, Nano Lett., 2014, 14(2), 676–681 CrossRef CAS PubMed.
- M. K. Zalalutdinov, J. T. Robinson, C. E. Junkermeier, J. C. Culbertson, T. L. Reinecke, R. Stine, P. E. Sheehan, B. H. Houston and E. S. Snow, Engineering Graphene Mechanical Systems, Nano Lett., 2012, 12(8), 4212–4218 CrossRef CAS PubMed.
- J. T. Robinson, J. S. Burgess, C. E. Junkermeier, S. C. Badescu, T. L. Reinecke, F. K. Perkins, M. K. Zalalutdniov, J. W. Baldwin, J. C. Culbertson, P. E. Sheehan and E. S. Snow, Properties of Fluorinated Graphene Films, Nano Lett., 2010, 10(8), 3001–3005 CrossRef CAS PubMed.
- H. Y. Liu, Z. F. Hou, C. H. Hu, Y. Yang and Z. Z. Zhu, Electronic and Magnetic Properties of Fluorinated Graphene with Different Coverage of Fluorine, J. Phys. Chem. C, 2012, 116(34), 18193–18201 CAS.
- Q. Li, X. Z. Liu, S.-P. Kim, V. B. Shenoy, P. E. Sheehan, J. T. Robinson and R. W. Carpick, Fluorination of Graphene Enhances Friction Due to Increased Corrugation, Nano Lett., 2014, 14(9), 5212–5217 CrossRef CAS PubMed.
- K. Davami, M. Shaygan, N. Kheirabi, J. Zhao, D. A. Kovalenko, M. H. Rümmeli, J. Opitz, G. Cuniberti, J.-S. Lee and M. Meyyappan, Synthesis and characterization of carbon nanowalls on different substrates by radio frequency plasma enhanced chemical vapor deposition, Carbon, 2014, 72, 372–380 CrossRef CAS.
- K. Davami, M. Shaygan and I. Bargatin, Fabrication of vertical graphene-based nanocomposite thin films, J. Mater. Res., 2015, 30, 617–625 CrossRef CAS.
- K. Davami, J. Cortes, N. Hong and B. Bargatin, Vertical graphene sheets as a lightweight light absorber, Mater. Res. Bull., 2016, 74, 226–233 CrossRef CAS.
- R. Stine, W.-K. Lee, K. E. Whitener, J. T. Robinson and P. E. Sheehan, Chemical Stability of Graphene Fluoride Produced by Exposure to XeF2, Nano Lett., 2013, 13(9), 4311–4316 CrossRef CAS PubMed.
- F. Yang and J. C. M. Li, Micro and Nano Mechanical Testing of Materials and Devices, Springer Science & Business Media, 2009 Search PubMed.
- S.-S. Lee, S.-W. Jang, K. Park, E. C. Jang, J.-Y. Kim, D. Neuhauser and S. Lee, A Mechanistic Study of Graphene Fluorination, J. Phys. Chem. C, 2013, 117(10), 5407–5415 CAS.
- B. Zhao, Y. Song, S. Wang, B. Dai, L. Zhang, Y. Dong, J. Lu and J. Hu, Mechanical mapping of nanobubbles by PeakForce atomic force microscopy, Soft Matter, 2013, 9, 8837–8843 RSC.
- C. R. Woods, L. Britnell, A. Eckmann, R. S. Ma, J. C. Lu, H. M. Guo, X. Lin, G. L. Yu, Y. Cao, R. V. Gorbachev, A. V. Kretinin, J. Park, L. A. Ponomarenko, M. I. Katsnelson, Y. N. Gornostyrev, K. Watanabe, T. Taniguchi and C. Casiraghi, Commensurate-incommensurate transition in graphene on hexagonal boron nitride, Nat. Phys., 2014, 10(6), 451–456 CrossRef CAS.
- S. Kalay, Y. Stetsyshyn, V. Lobaz, K. Harhay, H. Ohar and M. Çulha, Water-dispersed thermo-responsive boron nitride nanotubes: synthesis and properties, Nanotechnology, 2016, 27(3), 035703 CrossRef PubMed.
- A. Kostruba, M. Ohar, B. Kulyk, O. Zolobko and Y. Stetsyshyn, Surface modification by grafted sensitive polymer brushes: An ellipsometric study of their properties, Appl. Surf. Sci., 2013, 276, 340–346 CrossRef CAS.
- D. Maugis, Contact, Adhesion and Rupture of Elastic Solids, Springer Verlag, Berlin, 2000 Search PubMed.
- L. Yuan, Z. Y. Li, J. L. Yang and J. G. Hou, Diamondization of chemically functionalized graphene and graphene-BN bilayers, Phys. Chem. Chem. Phys., 2012, 14(22), 8179–8184 RSC.
- E. C. Junkermeier, S. C. Badescu and T. L. Reinecke, Highly Fluorinated Graphene, Condens. Matter, 2013, No. arXiv: 1302.6878 Search PubMed.
- S. Kwon, J. H. Ko, K. J. Jeon, Y. H. Kim and J. Y. Park, Enhanced Nanoscale Friction on Fluorinated Graphene, Nano Lett., 2012, 12(12), 6043–6048 CrossRef CAS PubMed.
- J.-H. Ko, S. Kwon, I. S. Byun, J. Choi, B. Park, Y.-H. Kim and J. Park, Nanotribological Properties of Fluorinated, Hydrogenated, and Oxidized Graphenes, Tribol. Lett., 2013, 50(2), 137–144 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra25068d |
| This journal is © The Royal Society of Chemistry 2016 |