Crosslinkable polyesters based on monomers derived from renewable lignin
Jie Zhang,
Chengcai Pang and
Guolin Wu*
Key Laboratory of Functional Polymer Materials, Institute of Polymer Chemistry, Nankai University, Tianjin 300071, PR China. E-mail: guolinwu@nankai.edu.cn
First published on 22nd January 2016
Abstract
Crosslinked polyesters were successfully prepared using thiol-ene click chemistry. Vanillic acid, syringic acid, and p-hydroxybenzoic acid have similar aromatic structures. They can be derived from a renewable resource, lignin. In this paper, they were used as raw materials for the synthesis of unsaturated thermoplastic polyesters, by polycondensation in solvent. The obtained polyesters were characterized by FTIR and NMR, and their tensile properties were tested. Compared to the other two unsaturated polyesters, the vanillic acid based unsaturated polyester (P1) was found to have the best tensile property. The latter was chosen for the preparation of the crosslinked polyester and further studies were carried out. Differential scanning calorimetry (DSC) revealed an amorphous character of P1, having a Tg of 35.8 °C. Thermogravimetric analysis (TGA) and tensile tests were conducted to study the thermal and mechanical properties of P1 and the crosslinked polyesters. The weight-average molecular weight (Mw) of P1 was found to be 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1. The unsaturated bonds in the polymers were found to have a special feature, which improved the flexibility of the polyesters on crosslinking. Moreover, both the unsaturated polyesters and the crosslinked polyesters were found to be biodegradable.
000 g mol−1. The unsaturated bonds in the polymers were found to have a special feature, which improved the flexibility of the polyesters on crosslinking. Moreover, both the unsaturated polyesters and the crosslinked polyesters were found to be biodegradable.
Introduction
Today, the vast majority of commercial synthetic polymeric materials (plastic, rubber, and fiber) are obtained from fossil fuels. These fossil resources are limited and their non-degradability leads to serious environmental pollution. This stimulates investigation, aimed at developing macromolecular materials from renewable feedstock that minimize the effects, detrimental to the environment, by their usage.1–4 Oils from plant or animal resources, represent a promising class of raw materials for polymer synthesis, owing to their abundance, inherent biodegradability, and versatility.5,6 The common renewable resources reported earlier are carbohydrates,7,8 plant-based oils,9,10 lignin,11 etc. Amongst the different types of polymeric systems, based on renewable resources, thermosetting resins are highly-crosslinked polymers that are cured by heat, pressure, light radiation, or a combination of these energy sources, and cannot be reshaped after curing.12,13Synthesis of polyesters from renewable resources has been an appealing topic in recent years, owing to the ecological and economical aspects.14 It is well known that lignin is a renewable material that has the potential to yield valuable single aromatic chemicals when strategically depolymerized, and it is the second most abundant natural raw material, only after cellulose.15 Vanillin, derived from lignin, has been identified as a suitable bio-based replacement for use in vinyl ester resins, mainly due to its aromatic character. The incorporation of aromatic groups into cured resins can provide structural rigidity and thermal stability by inhibiting the rotational freedom of the polymeric network.16,17 As an attractive bio-based monomer, vanillin has been successfully incorporated into novel polymers for use in a wide variety of commercial applications.18 Moreover, syringic acid and p-hydroxybenzoic acid, having structures similar to vanillin, are also potential bio-based monomers derived from lignin.
Click chemistry reactions are often cited as chemical syntheses that are consistent with the goals of green chemistry.19 Crosslinking, brought about by UV or visible light, is a widespread and convenient approach, leading to enhancement in thermal and mechanical properties of the bio-based unsaturated polyesters.14 Thiol-ene chemistry has developed in the past few decades and has been identified as a ‘click’ method, due to its high conversions and low side products.20 Thiol-ene coupling of various polymeric enes has also been extensively studied.21 The unsaturated bond in the polyester monomer allows these polymers to be crosslinked.22 Crosslinking is a very important process in the formation of elastomeric films, wherein the formed network determines the applications and properties of the film.23,24 The three-dimensional structures of the networks and the final properties of the films depend not only on the type and content of crosslinking agents but also on the ratio of thiol to double bond, which is a crucial parameter.25
In our previous studies, several saturated polyesters using vanillic acid were synthesized.29,30 In the present study, three unsaturated polyesters using vanillic acid, syringic acid, and p-hydroxybenzoic acid have been synthesized under mild reaction conditions. 2,2′-(Ethylenedioxy)diethanethiol has been used as the crosslinker to obtain thin crosslinked films, after irradiation in solution state by UV light, in the presence of a photoinitiator, (2,2-dimethoxy-2-phenylacetophenone). They have been found to have better tensile properties. Furthermore, different strategies to obtain modified aromatic polyesters, which can be either chemically degraded under smooth conditions or biologically, are currently drawing a lot of interest.26,27 Most of the engineering plastics that are obtained from synthetic polymers, are non-biodegradable.28 In this work, the degradation of bio-based polyesters has also been investigated in different media.
Experimental section
1. Materials
Vanillic acid (4-hydroxy-3-methoxybenzoic acid, 98%), syringic acid (98%) and p-hydroxybenzoic acid (98%) were purchased from Shanghai Darui Fine Chemical Co. Ltd. Trans-1,4-dibromo-2-butene (98%), and 2,2′-(ethylenedioxy)diethanethiol (95%) were obtained from Heowns (Tianjin, China). Potassium carbonate (99%), 2,2′-dimethoxy-2-phenylacetophenone (DMPA, 98%), 1-methyl-2-pyrroline (99%), chloroform (99%), methanol anhydrous (99.5%), citric acid (99.5%), and trisodium citrate dehydrate (99%) were supplied by Tianjin Chemical Reagent Co. Ltd. Lipase from porcine pancreas was purchased from Sigma-Aldrich. Methanol, 1-methyl-2-pyrroline and other solvents were dried according to the standard methods in literature.312. General instrumentation and methods
NMR spectra were recorded in CDCl3 at 25 °C, using a Bruker AC-400 NMR spectrometer. Tetramethylsilane was used as the internal standard. Fourier transform infrared spectra (FTIR) were measured at room temperature using a Bio-Rad FTS6000 spectrophotometer. Each polymer sample for FTIR was prepared by grinding the polymer adequately with KBr powder, followed by compressing the mixture to form a pellet. The molecular weights and molecular weight distributions of the obtained polymers were determined by gel permeation chromatography (GPC). THF was used as the eluent at a flow rate of 1.0 mL min−1. The average molecular weights were calibrated against monodisperse polystyrene (PS) standards. Thermogravimetric analysis (TGA) was performed using a Seiko Exstar 6000 TGA quartz rod microbalance. As a general method, the polymer sample was heated from 27 to 800 °C at a heating rate of 10 °C min−1. The temperature at which 5% weight loss occurred and the temperature at which maximum rate of degradation took place were recorded. Differential scanning calorimetric (DSC) studies were carried out using DSC Q100 from TA instruments. Polymer samples were first heated from −50 °C to 150 °C and the glass transition temperature (Tg) was determined in the second heating run. All runs were carried out at a rate of 10 °C min−1. The tensile assays were conducted in triplicates on rectangular samples having the dimensions (length × width × thickness = 12 mm × 2 mm × 0.5 mm). The strain was measured by applying a ramp rate of 0.5 N min−1 at 25 °C. A preload force of 0.05 N and a soak time of 3 min were employed. All films for tensile tests were obtained by casting chloroform solutions having concentrations of 0.1 g mL−1.3. General procedure for the preparation of unsaturated polyesters
The unsaturated polyesters were prepared according to a procedure mentioned in the literature.32 Trans-1,4-dibromo-2-butene (8.56 g, 0.04 mol) was dissolved in 100 mL 1-methyl-2-pyrroline, containing vanillic acid (6.72 g, 0.04 mol) (or 0.04 mol syringic acid, or 0.04 mol p-hydroxybenzoic acid) in a three-neck round-bottom flask, wrapped by a foil, by stirring at 40 °C. Potassium carbonate (11 g, 0.08 mol) was added to the clear solution and the mixture was refluxed for 60 hours under N2 atmosphere. The viscous solution was diluted with THF and poured into a large excess of cold water, which was acidified by adding a few drops of concentrated HCl. The precipitated polymer was filtered and then washed repeatedly with distilled water till neutral pH, then washed with methanol and finally dried under vacuum till the product attained a constant weight. The yields of the products were more than 90%.4. Preparation of the crosslinked thermoplastic polyesters
The crosslinked thermoplastic polyesters were obtained by mixing 1 g unsaturated vanillic acid-based polyester, containing 4.5 mmol C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, with a specific amount of 2,2′-(ethylenedioxy)diethanethiol (0.9, 1.8, or 2.7 mmol) as the crosslinking agent, in chloroform, along with 5% DMPA as the photoinitiator. The reaction was irradiated with UV light (λ = 365 nm, 400 W). The crosslinking process was monitored by infrared spectroscopy.
C, with a specific amount of 2,2′-(ethylenedioxy)diethanethiol (0.9, 1.8, or 2.7 mmol) as the crosslinking agent, in chloroform, along with 5% DMPA as the photoinitiator. The reaction was irradiated with UV light (λ = 365 nm, 400 W). The crosslinking process was monitored by infrared spectroscopy.
5. In vitro degradation
Vanillic acid-based unsaturated polyester and crosslinked vanillic acid-based polyester films, having thicknesses of 0.5 mm, were prepared by casting their chloroform solutions (C = 0.1 g mL−1). The films were cut into specimens, each weighing 25 mg, and dried under vacuum till their weights were constant. Rate of degradation after hydrolysis were measured by incubating the crosslinked polyester films in 20 mL of citric acid buffer solution at pH 2.5 at 80 °C, with continuous shaking (60 strokes per min). After specified periods of time, the samples were rinsed thoroughly with distilled water and dried till their weights were constant, under vacuum at 50 °C, and reweighed to determine the mass losses. Meanwhile, the enzymatic degradations were carried out at 37 °C in pH 7.4 PBS buffered solutions containing lipase from porcine pancreas (1 mg mL−1). P1 and crosslinked P1 with 20% crosslinker were tested. The buffered enzyme solutions were replaced every 72 h to maintain the enzyme activity. A sample immersed in the phosphate solution, without lipase, was used as a control. GPC and NMR spectroscopy were used to analyze the degradation products.Results and discussion
1. Synthesis of polyesters
The purpose of this work is to prepare a crosslinkable polyester chemically, and then to improve its mechanical properties by crosslinking. In the first step, vanillic acid, syringic acid, and p-hydroxybenzoic acid are used as the starting materials, each of which is reacted with trans-1,4-dibromo-2-butene, to prepare the unsaturated polyesters, P1, P2, and P3, respectively (Scheme 1). All the three raw materials are obtained from renewable resources. The GPC analyses indicate that the vanillic acid based polyester has a weight average molecular weight (Mw) of 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and number average molecular weight (Mn) of 44
000 g mol−1 and number average molecular weight (Mn) of 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1, with a dispersity of 1.6. Syringic acid and p-hydroxybenzoic acid based polyesters have Mw of 12
600 g mol−1, with a dispersity of 1.6. Syringic acid and p-hydroxybenzoic acid based polyesters have Mw of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 11
000 and 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, with dispersities of 1.51 and 1.47, respectively. The obtained unsaturated polyesters are characterized by 1H-NMR, 13C-NMR, and FT-IR (Fig. 1–3). Fig. 1 shows the 1H-NMR spectra of the three unsaturated polyesters. All three polymers show peaks at 4.8 ppm, 4.7 ppm, and 6.0 ppm. The appearance of two singlets at 4.8 ppm and 4.7 ppm suggests the formation of the ester and ether-linkages. The triplet at 6.0–6.1 ppm is attributed to the protons on the unsaturated bond (–C
000 g mol−1, with dispersities of 1.51 and 1.47, respectively. The obtained unsaturated polyesters are characterized by 1H-NMR, 13C-NMR, and FT-IR (Fig. 1–3). Fig. 1 shows the 1H-NMR spectra of the three unsaturated polyesters. All three polymers show peaks at 4.8 ppm, 4.7 ppm, and 6.0 ppm. The appearance of two singlets at 4.8 ppm and 4.7 ppm suggests the formation of the ester and ether-linkages. The triplet at 6.0–6.1 ppm is attributed to the protons on the unsaturated bond (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–) in the copolymers. Fig. 2 shows the 13C-NMR spectrum of P1. The linear polyester shows the expected main signals from the chemical structure of the repeating unit. The two methylene carbons, involved in the ether (–CH2–O–Ar) and ester (–COOCH2–) groupings are well separated. The signal corresponding to the unsaturated carbons (–C
C–) in the copolymers. Fig. 2 shows the 13C-NMR spectrum of P1. The linear polyester shows the expected main signals from the chemical structure of the repeating unit. The two methylene carbons, involved in the ether (–CH2–O–Ar) and ester (–COOCH2–) groupings are well separated. The signal corresponding to the unsaturated carbons (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–) appears between 129.0–129.6 ppm, and the ester carbonyl (–COO–) appears at 165.9 ppm. Fig. 3 shows the FT-IR spectra of the three unsaturated polyesters. The three unsaturated polyesters show similar FTIR absorption patterns. In the FTIR spectra, the stretching vibrations of the ester carbonyl, attached to the aromatic ring, appears at 1713 cm−1. The absorption peaks at 1670 cm−1 and 1600 cm−1 are attributed to the unsaturated bond (C
C–) appears between 129.0–129.6 ppm, and the ester carbonyl (–COO–) appears at 165.9 ppm. Fig. 3 shows the FT-IR spectra of the three unsaturated polyesters. The three unsaturated polyesters show similar FTIR absorption patterns. In the FTIR spectra, the stretching vibrations of the ester carbonyl, attached to the aromatic ring, appears at 1713 cm−1. The absorption peaks at 1670 cm−1 and 1600 cm−1 are attributed to the unsaturated bond (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), and the aromatic rings in the polymer chains, respectively.
C), and the aromatic rings in the polymer chains, respectively.
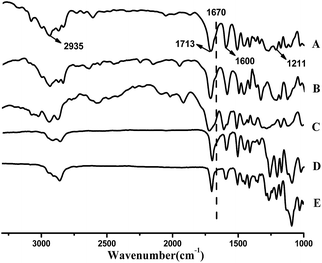 | ||
| Fig. 3 FTIR spectra of P1, P2, P3 and crosslinked P1 with different degree of crosslinking. A: P1; B: P2; C: P3; D: crosslinked P1 with 20% crosslinker; E: crosslinked P1 with 60% crosslinker. | ||
It is worth noting that all the three monomers, derived from biomass, having unsymmetrical structures. These monomers have two functional groups, phenolic hydroxyl and carboxyl. Both these groups can react with trans-1,4-dibromo-2-butene under alkaline conditions, producing aregic polymers. For example, in the case of vanillic acid, two orientations of vanillic acid are possible along the polymeric chain, leading to two isoregic and two syndioregic dyad sequences, as illustrated in Fig. 4. As it could be reasonably anticipated, four different peaks would appear in the 13C-NMR spectrum, arising from the methylene adjacent to the unsaturated bond (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–), which correspond to the four magnetically different CH2–Os (two from the iso dyads and two from the syndio dyads). Measurement of the peak areas shows that P1 is composed of 45.5% isoregic and 54.5% syndioregic dyads. Therefore, it is evident that essentially the vanillic acid units are randomly incorporated into the chain. The other two bio-based monomers show similar trends.
C–), which correspond to the four magnetically different CH2–Os (two from the iso dyads and two from the syndio dyads). Measurement of the peak areas shows that P1 is composed of 45.5% isoregic and 54.5% syndioregic dyads. Therefore, it is evident that essentially the vanillic acid units are randomly incorporated into the chain. The other two bio-based monomers show similar trends.
2. Tensile properties
The mechanical properties of the three polyesters derived from renewable resources, vanillic acid, syringic acid and p-hydroxybenzoic acid were investigated. The recorded stress–strain curves for the obtained unsaturated polyesters are shown in Fig. 5. It would not be expedient to directly compare the mechanical properties of these polymers. The unsaturation in the polyester allows the polymer to crosslink. The second step involves the preparation of the crosslinked polyesters. P1 was selected for the preparation of the crosslinked polyesters, through thiol-ene click chemistry, since it showed a better tensile property, compared to the other two unsaturated polyesters (Scheme 1). The reduction in the intensity of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C absorption peak at 1670 cm−1 in the FT-IR spectra of crosslinked polyesters suggests a chemical crosslinking reaction, as shown in Fig. 3. The most obvious attribute of a crosslinked polyester material, reported here, is its tensile property. The stress–strain curves of the crosslinked polyesters with different degrees of crosslinking are shown in Fig. 5 and the mechanical property parameters are presented in Table 1. Initially, with a concentration of crosslinker below 40%, the ultimate strength increases as the degree of crosslinking increases. However, as the concentration of crosslinker crosses 40%, the ultimate strength decreases with an increase in the degree of crosslinking. This can be explained on the basis of a change in the crosslined structure. After crosslinking, the polyester forms a 3D network, which inhibits the activity of the polymeric chains and the deformation capacity of the materials. Therefore, the results show an increase of stress, and a decrease of strain. The strain at break for unsaturated polyester P1 is 495%, whereas the values are 242%, 290%, and 240% for the crosslinked polymers with 20%, 40%, and 60% crosslinkers, respectively. However, if the crosslink density is too high, the crosslinked polyesters generate more uneven networks. Under the influence of external force, the loose part in the network is destroyed first, which induces a decrease in the measured strength. Amongst the samples, the crosslinked polyester with 40% crosslinker has the highest ultimate stress of 9.56 MPa. The Young's modulus for P1 is 9.03 MPa, whereas the values are 4.6, 8.57, and 13.6 MPa for crosslinked P1 with 20%, 40%, and 60% crosslinkers, respectively. After crosslinking, the polyester becomes elastomeric and the Young's modulus decreases, when the concentration of crosslinker is below 40%. When the concentration of crosslinker is 60%, the value of elongation at break is minimal; the stress improves to a certain degree and has the maximum Young's modulus. This result shows that the degree of crosslinking affects the deformation degree. It can be concluded that optimum crosslinking can improve the characteristic tensile properties.
C absorption peak at 1670 cm−1 in the FT-IR spectra of crosslinked polyesters suggests a chemical crosslinking reaction, as shown in Fig. 3. The most obvious attribute of a crosslinked polyester material, reported here, is its tensile property. The stress–strain curves of the crosslinked polyesters with different degrees of crosslinking are shown in Fig. 5 and the mechanical property parameters are presented in Table 1. Initially, with a concentration of crosslinker below 40%, the ultimate strength increases as the degree of crosslinking increases. However, as the concentration of crosslinker crosses 40%, the ultimate strength decreases with an increase in the degree of crosslinking. This can be explained on the basis of a change in the crosslined structure. After crosslinking, the polyester forms a 3D network, which inhibits the activity of the polymeric chains and the deformation capacity of the materials. Therefore, the results show an increase of stress, and a decrease of strain. The strain at break for unsaturated polyester P1 is 495%, whereas the values are 242%, 290%, and 240% for the crosslinked polymers with 20%, 40%, and 60% crosslinkers, respectively. However, if the crosslink density is too high, the crosslinked polyesters generate more uneven networks. Under the influence of external force, the loose part in the network is destroyed first, which induces a decrease in the measured strength. Amongst the samples, the crosslinked polyester with 40% crosslinker has the highest ultimate stress of 9.56 MPa. The Young's modulus for P1 is 9.03 MPa, whereas the values are 4.6, 8.57, and 13.6 MPa for crosslinked P1 with 20%, 40%, and 60% crosslinkers, respectively. After crosslinking, the polyester becomes elastomeric and the Young's modulus decreases, when the concentration of crosslinker is below 40%. When the concentration of crosslinker is 60%, the value of elongation at break is minimal; the stress improves to a certain degree and has the maximum Young's modulus. This result shows that the degree of crosslinking affects the deformation degree. It can be concluded that optimum crosslinking can improve the characteristic tensile properties.
 | ||
| Fig. 5 Stress–strain curves at 25 °C, 50 mm min−1. A: P1; B: P2; C: P3; D: crosslinked P1 with 20% crosslinker; E: crosslinked P1 with 40% crosslinker; F: crosslinked P1 with 60%. | ||
| Polymer | Young's modulus (MPa) | Ultimate strength (MPa) | Strain at break (%) |
|---|---|---|---|
| P1 | 9.03 ± 0.93 | 1.4 ± 0.06 | 495 ± 37 |
| Crosslinked P1 with 20% crosslinker | 4.6 ± 0.48 | 2.13 ± 0.04 | 242 ± 33 |
| Crosslinked P1 with 40% crosslinker | 8.57 ± 1.05 | 9.56 ± 0.3 | 291 ± 12 |
| Crosslinked P1 with 60% crosslinker | 13.6 ± 0.9 | 5.56 ± 0.11 | 240 ± 21 |
| P2 | 1.44 ± 0.04 | 0.71 ± 0.07 | 452 ± 51 |
| P3 | 3.36 ± 0.12 | 1.41 ± 0.06 | 461 ± 27 |
3. Thermal properties
DSC analyses reveal the amorphous character of P1. The Tg for P1 is 35.8 °C, as shown in Fig. 6. The thermal stabilities of P1 and its crosslinked materials are investigated using TGA. The data is presented in Table 2. Fig. 7 shows that after crosslinking, the initial degradation temperature of the polymer decreases. The T5% for P1 is 318 °C, and 180 °C and 194 °C for crosslinked P1 with 40% and 60% crosslinker, respectively. The crosslinked materials degrade sooner than the polyester precursors, because much weaker C–S bonds are introduced between the chains, as a result of the crosslinking reaction. This characteristic feature has been reported earlier.33,344. In vitro degradation
It is well known that normally aromatic polyesters, hydrolyze and biodegrade with difficulty.26 To investigate the biodegradability of bio-based polyester P1, it was incubated in pH 7.4 buffer at 37 °C, with and without porcine pancreas lipase. The changes in the weights of the samples and molecular weight of P1 at the end of the incubation time are shown in the Table 3. The biodegradation was more noticeable when lipase was present in the medium. In buffered sodium phosphate solution, having pH 7.4 and lipase, P1 loses more than 50% of its initial weight after 100 days and the Mn and Mw also showed a decrease by more than 65%. In case of crosslinked P1 with 20% crosslinker, the samples were incubated at 37 °C in buffered sodium phosphate solution with porcine pancreas lipase. The average time for losing 50% of its weight was about 120 days.| Buffer solution | Reaction condition | m0a (mg) | mea (mg) | Mwb | Mnb | Time (days) |
|---|---|---|---|---|---|---|
| a m0: initial weight of the samples; me: remaining weight after degradation.b Mw: Mw after degradation; Mn: Mn after degradation. | ||||||
| pH 2.5 | 80 °C | 19.5 | 9.6 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 566 566 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 103 103 |
20 |
| 20 °C | 25.7 | 12.6 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 756 756 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 831 831 |
100 | |
| pH 7.4 | Include lipase (37 °C) | 26.4 | 12.5 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 552 552 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 106 106 |
100 |
| Without lipase (37 °C) | 24.7 | 23.5 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 627 627 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 934 934 |
100 | |
To test the hydrodegradability of these polyesters in a more aggressive medium, P1 and crosslinked P1 polyesters were incubated at 80 °C and 20 °C in citric acid buffer at pH 2.5. The sample P1 incubated at 80 °C lost more than 50% only in 20 days, whereas the sample incubated at 20 °C required 100 days to lose the same amount of weight. This implies that high temperature and acidic pH enhance the rate of degradation. In the case of crosslinked P1 with 60% crosslinker, when the samples were incubated at 80 °C, the average time for weight loss of more than 50% was 85 days. However, in the case of crosslinked P1 with 20% crosslinker, the time required was 42 days. This implies that the extent of crosslinking can affect the rate of degradation of the crosslinked polymer. The degradation curves of unsaturated and crosslinked polyesters are shown in Fig. 8.
 | ||
| Fig. 8 Degradation curves of P1 and crosslinked P1 under 80 °C in citric acid buffer. A: P1; B: crosslinked P1 with 20% crosslinker; C: crosslinked P1 with 60% crossliner. | ||
NMR study was undertaken to have an insight of the degradation of the polyester chains at a molecular level. 1H NMR spectra of P1 incubated in citric acid buffer at 80 °C and 20 °C and the one incubated in buffered sodium phosphate solution, with and without lipase, are all shown in Fig. 9. Spectra of P1 in citric acid buffer at 80 °C and in buffered sodium phosphate solution with lipase show appearance of a new peak at 1.6 ppm, when compared to the initial spectra. This shows that degradation of this polyester occurs mainly due to the splitting of the unsaturated ester groups in the polyester.
Conclusions
Three unsaturated polyesters were synthesized from vanillic acid, syringic acid, and p-hydroxybenzoic acid derived from lignin. The monomers had one, two and zero methoxy groups on the benzene ring, respectively. Comparison of the properties of the three unsaturated polyesters showed that the polyester synthesized from vanillic acid had the highest molecular weight and best tensile mechanical properties. A series of crosslinked elastomeric polyesters were prepared by the thiol-ene click reaction, using 2,2′-(ethylenedioxy)diethanethiol as the crosslinking agent. The experimental data and results showed that an appropriate degree of crosslinking could improve the tensile property effectively. With 40% crosslinker, the ultimate strength reached 9.56 MPa, and strain at break was 290%. The integrated mechanical properties were increased, compared to the unsaturated polyester and to that of the previous work. The crosslinked elastomeric polyesters showed steady thermal properties. They could be degraded in citric acid buffer at pH 2.5 and in buffered sodium phosphate solution, containing porcine pancreas lipase, at pH 7.4. Compared to other plastic materials widely used commercially, the polyesters synthesized from renewable materials have an enormous advantage of being biodegradable, which can decrease environmental pollution to a great extent.Acknowledgements
This work was funded by NSFC (51203079), the Natural Science Foundation of Tianjin (14JCYBJC18100), and PCSIRT (IRT1257).References
- R. D. Ashby, D. K. Y. Solaiman, G. D. Strahan, C. Zhu, R. C. Tappel and C. T. Nomura, Bioresour. Technol., 2012, 118, 272–280 CrossRef CAS PubMed.
- M. Jiang, Q. Liu, Q. Zhang, C. Ye and G. Zhou, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1026–1036 CrossRef CAS.
- M. J. L. Tschan, E. Brulé, P. Haquette and C. M. Thomas, Polym. Chem., 2012, 3, 836–851 RSC.
- C. Vilela, A. F. Sousa, A. C. Fonseca, A. C. Serra, J. F. J. Coelho, C. S. R. Freire and A. J. D. Silvestre, Polym. Chem., 2014, 5, 3119–3141 RSC.
- N. Supanchaiyamat, A. J. Hunt, P. S. Shuttleworth, C. Ding, J. H. Clark and A. S. Matharu, RSC Adv., 2014, 4, 23304–23313 RSC.
- C. Aouf, E. Durand, J. Lecomte, M.-C. Figueroa-Espinoza, E. Dubreucq, H. Fulcrand and P. Villeneuve, Green Chem., 2014, 16, 1740–1754 RSC.
- C. Lavilla, A. M. de Ilarduya, A. Alla, M. G. García-Martín, J. A. Galbis and S. Muñoz-Guerra, Macromolecules, 2012, 45, 8257–8266 CrossRef CAS.
- C. Japu, A. Alla, A. Martínez de Ilarduya, M. G. García-Martín, E. Benito, J. A. Galbis and S. Muñoz-Guerra, Polym. Chem., 2012, 3, 2092–2101 RSC.
- J. Hong, B. K. Shah and Z. S. Petrović, Eur. J. Lipid Sci. Technol., 2013, 115, 55–60 CrossRef CAS.
- L. Maisonneuve, T. Lebarbé, E. Grau and H. Cramail, Polym. Chem., 2013, 4, 5472–5517 RSC.
- T. Saito, R. H. Brown, M. A. Hunt, D. L. Pickel, J. M. Pickel, J. M. Messman, F. S. Baker, M. Keller and A. K. Naskar, Green Chem., 2012, 14, 3295–3303 RSC.
- J. M. Raquez, M. Deléglise, M. F. Lacrampe and P. Krawczak, Prog. Polym. Sci., 2010, 35, 487–509 CrossRef CAS.
- T. Lebarbé, L. Maisonneuve, T. H. Nga Nguyen, B. Gadenne, C. Alfos and H. Cramail, Polym. Chem., 2012, 3, 2842–2851 RSC.
- L. Jasinska and C. E. Koning, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 2885–2895 CrossRef CAS.
- L. Mialon, R. Vanderhenst, A. G. Pemba and S. A. Miller, Macromol. Rapid Commun., 2011, 32, 1386–1392 CrossRef CAS PubMed.
- J. F. Stanzione III, J. M. Sadler, J. J. La Scala, K. H. Reno and R. P. Wool, Green Chem., 2012, 14, 2346–2352 RSC.
- J. D. P. Araújo, C. A. Grande and A. E. Rodrigues, Chem. Eng. Res. Des., 2010, 88, 1024–1032 CrossRef.
- J. F. Stanzione 3rd, J. M. Sadler, J. J. La Scala and R. P. Wool, ChemSusChem, 2012, 5, 1291–1297 CrossRef PubMed.
- G. Lligadas, J. C. Ronda, M. Galià and V. Cádiz, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 2111–2124 CrossRef CAS.
- M. Claudino, I. van der Meulen, S. Trey, M. Jonsson, A. Heise and M. Johansson, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 16–24 CrossRef CAS.
- O. Türünç and M. A. R. Meier, Eur. J. Lipid Sci. Technol., 2013, 115, 41–54 CrossRef.
- A. Llevot, E. Grau, S. Carlotti, S. Grelier and H. Cramail, Polym. Chem., 2015, 6, 7693–7700 RSC.
- Y. Jiang, G. O. R. A. van Ekenstein, A. J. J. Woortman and K. Loos, Macromol. Chem. Phys., 2014, 215, 2185–2197 CrossRef CAS.
- P. A. Wilbon, F. Chu and C. Tang, Macromol. Rapid Commun., 2013, 34, 8–37 CrossRef CAS PubMed.
- M. Desroches, S. Caillol, R. Auvergne, B. Boutevin and G. David, Polym. Chem., 2012, 3, 450–457 RSC.
- C. Lavilla, A. Alla, A. Martínez de Ilarduya, E. Benito, M. G. García-Martín, J. A. Galbis and S. Muñoz-Guerra, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 3393–3406 CrossRef CAS.
- S. A. Madbouly, J. A. Schrader, G. Srinivasan, K. Liu, K. G. McCabe, D. Grewell, W. R. Graves and M. R. Kessler, Green Chem., 2014, 16, 1911–1920 RSC.
- W. Dong, J. Ren, L. Lin, D. Shi, Z. Ni and M. Chen, Polym. Degrad. Stab., 2012, 97, 578–583 CrossRef CAS.
- C. Pang, J. Zhang, G. Wu, Y. Wang, H. Gao and J. Ma, Polym. Chem., 2014, 5, 2843–2853 RSC.
- C. Pang, J. Zhang, Q. Zhang, G. Wu, Y. Wang and J. Ma, Polym. Chem., 2015, 6, 797–804 RSC.
- W. L. F. Armarego and C. L. L. Chai, Purification of Laboratory Chemicals, Butterworth-Heineman Ltd., 7th edn, 2013, pp. 180–462 Search PubMed.
- A. Parthiban, F. Ming Choo and C. L. L. Chai, Polym. Int., 2011, 60, 1624–1628 CrossRef CAS.
- R. K. Bharadwaj, A. R. Mehrabi, C. Hamilton, C. Trujillo, M. Murga, R. Fan, A. Chavira and A. K. Thompson, Polymer, 2002, 43, 3699–3705 CrossRef CAS.
- P.-Y. Kuo, M. Sain and N. Yan, Green Chem., 2014, 16, 3483–3493 RSC.
| This journal is © The Royal Society of Chemistry 2016 |







