Heat stability improvement of whey protein isolate via glycation with maltodextrin without control of the relative humidity
Abstract
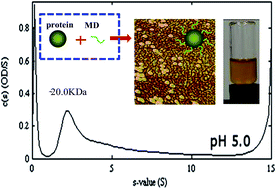
The reduction of protein aggregation and their improved heat stability in solutions are often achieved through glycation. While a controlled relative humidity (RH) is standard practice in the glycation process, its requirement and associated cost, as a condition of the treatment, has not been demonstrated. The improved heat stability of whey protein isolate (WPI) at pH 3–7 and 0–150 mM NaCl after glycation with maltodextrin (MD) using the Maillard reaction in the dry state without the need to control the RH is reported in this study. Dispersions of glycated WPI remained transparent after heating at 88 °C for 2 min at pH values that are close to whey protein pI values, including pH 5.0. Transparent dispersions were enabled as indicated by circular dichroism of the WPI–MD conjugates heated in aqueous solutions that underwent secondary structure changes and by AFM images that indicated globular aggregates smaller than 40 nm.

 Please wait while we load your content...
Please wait while we load your content...