Metal-redox for MnAl-Based ternary magnetic nanocrystals†
Abstract
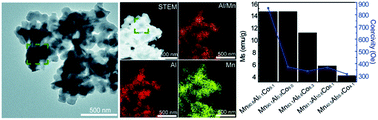
We report the unique metal-redox nanosynthesis of ligand-free MnAl nanocrystals for the first time, where the doped cobalt element is shown to stabilize metastable tetragonal MnAl. The magnetic characteristics of MnAl nanocrystals can be tuned by the atomic percentage of cobalt dopant. The ternary CoMnAl nanoalloys have a potential application in the critical-energy related magnet application.

 Please wait while we load your content...
Please wait while we load your content...