DOI:
10.1039/C5RA26117A
(Paper)
RSC Adv., 2016,
6, 22896-22907
The effects of light and temperature on microalgal growth and nutrient removal: an experimental and mathematical approach†
Received
7th December 2015
, Accepted 20th February 2016
First published on 22nd February 2016
Abstract
Cultivation of microalgae and cyanobacteria has been intensified in the last decades, due to the numerous applications described for these microorganisms. However, the high process costs associated with biomass production systems reduce the economic feasibility of microalgal/cyanobacterial cultivation. A better understanding of the effects of light and temperature on growth kinetics will contribute to the improvement of biomass productivities and reduce the costs associated with the optimization of culture parameters. In this study, the effects of average daily light irradiance and temperature on growth and nutrient removal were assessed using Chlorella vulgaris, Pseudokirchneriella subcapitata, Synechocystis salina and Microcystis aeruginosa. Additionally, a mathematical model relating specific growth rates with these variables was developed. Both kinetic growth parameters and nutrient removal had similar responses to light and temperature: increasing light supply, higher specific growth rates, biomass productivities and nutrient removal efficiencies were achieved. Among the studied temperatures, all microorganisms presented higher biomass productivities and nutrient removal efficiencies at 25 °C. Regarding the results from the mathematical model, the optimal temperature for the selected microorganisms was 25.3 ± 1.1 °C. On the other hand, the optimal average daily light irradiances varied with the species, being 208, 258, 178 and 140 μE m−2 s−1 for C. vulgaris, P. subcapitata, S. salina and M. aeruginosa, respectively.
1. Introduction
Microalgae are a broad category of photosynthetic microorganisms, comprising single-cell eukaryotic microalgae and prokaryotic cyanobacteria. Cultivation of these photosynthetic microorganisms has gained much attention in the last decades, due to the huge potential of these microorganisms in such a variety of applications. When growing autotrophically, microalgae and cyanobacteria uptake CO2 from the atmosphere and/or flue gas emissions, reducing the concentration of this greenhouse gas in the atmosphere.1 Additionally, these microorganisms assimilate nitrogen and phosphorus, the main contributors to the eutrophication phenomenon, playing an important role in the remediation of water resources.2,3 Due to the rich composition of microalgal/cyanobacterial cells, their biomass can then be used in different applications, such as human food and animal feed, production of drugs, cosmetics, functional food, biofuels and fertilizers.4–7 Despite the numerous applications described for microalgae and cyanobacteria, cultivation of these microorganisms still presents some challenges regarding the achievement of high biomass productivities at reduced costs. Accordingly, optimization of cultivation parameters in order to obtain an economically viable process with increased biomass productivities becomes necessary. Microalgal/cyanobacterial growth can be affected by several factors, both biotic and abiotic. Biotic factors include the presence of pathogens, such as bacteria, fungi and viruses, and the competition by other microalgae, whereas abiotic factors include light, temperature, pH, salinity, nutrient qualitative and quantitative profiles, dissolved oxygen concentration and the presence of toxic compounds. Additionally, microalgal and cyanobacterial growth can be influenced by operational conditions, such as hydraulic residence time, harvesting rates, gas transfer and mixing.8–11 Among these parameters, light supply and temperature appear as the most important factors influencing microalgal and cyanobacterial growth. In fact, photoautotrophic growth is driven by light supply, the energy source that is used to convert inorganic carbon into organic matter, and changes in temperature can easily affect microalgal/cyanobacterial growth since the metabolic activity of these photosynthetic microorganisms can be ceased by extreme temperatures. Furthermore, interaction between these variables in outdoor cultures determines the biochemical profile of the resulting biomass and growth state.12
In this study, the effects of light supply (average daily light irradiance) and temperature on biomass production and nutrient uptake were assessed for the microalgae Chlorella vulgaris and Pseudokirchneriella subcapitata and the cyanobacteria Synechocystis salina and Microcystis aeruginosa. Selection of these microorganisms was based on the following factors:13–16 (i) these microalgae and cyanobacteria can be easily grown in laboratory cultures; and (ii) several authors have reported the use of these microorganisms in a wide variety of biotechnological applications, such as CO2 capture, wastewater treatment, biofuels production and synthesis of bioactive compounds. Additionally, due to the wide diversity of microalgal and cyanobacterial species, the study and optimization of culture parameters for all these microorganisms under different light and temperature conditions is very difficult. In this sense, mathematical modelling of these variables constitutes an important tool for growth prediction and characterization. Mathematical models describing the effect of light supply and temperature on microalgal/cyanobacterial growth have already been reported in the literature.17–20 However, only a few studies have considered both variables simultaneously.21–23 Accordingly, a kinetic growth model was developed to determine optimal light and temperature conditions for the selected microorganisms.
2. Materials and methods
2.1. Microorganisms and culture medium
The microalgae C. vulgaris CCAP 211/11B and P. subcapitata CCAP 278/4 were obtained from Culture Collection of Algae and Protozoa (United Kingdom), while the cyanobacteria S. salina LEGE 06079 and M. aeruginosa LEGE 91344 were obtained from the Laboratory of Ecotoxicology, Genomic and Evolution – CIIMAR (Centre of Marine and Environmental Research of the University of Porto, Portugal). Stock solutions of these microorganisms were prepared in OECD (Organisation for Economic Co-operation and Development) test medium,24 with the following composition (per litre): 15 mg NaNO3, 12 mg MgCl2·6H2O, 18 mg CaCl2·2H2O, 15 mg MgSO4·7H2O, 1.6 mg KH2PO4, 0.08 mg FeCl3·6H2O, 0.1 mg Na2EDTA·2H2O, 0.185 mg H3BO3, 0.415 mg MnCl2·4H2O, 3 μg ZnCl2, 1.5 μg CoCl2·6H2O, 0.01 μg CuCl2·2H2O, 7 μg Na2MoO4·2H2O and 50 mg NaHCO3. The cells were incubated in 500 mL flasks at room temperature, under continuous fluorescent light with an irradiance of 120 μE m−2 s−1 (corresponding average daily light irradiance is 120 μE m−2 s−1) at the surface of the flasks. Agitation was obtained by bubbling atmospheric air (filtered through 0.22 μm cellulose acetate membranes, Orange Scientific, Belgium) at the bottom of the flasks.
2.2. Experimental setup and cultivation conditions
Batch experiments were performed in 500 mL flasks (VWR, Portugal) with a working volume of 400 mL. As the growth medium described above presents a very low concentration of nitrogen and phosphorus, concentrations of these elements were increased to simulate the concentrations commonly present in a secondary treated effluent. Therefore, cells were cultivated for 12 days in the culture medium described above, but with the following concentrations of NaNO3 and KH2PO4: 250 and 45 mg L−1, respectively.25 In this study, nitrate was used as nitrogen source because this is the most thermodynamically stable form of inorganic nitrogen8 and also because it is the most abundant nitrogen form in the tertiary treatment step of wastewater treatment plants, where microalgae can play an important remediation role.25 The experimental conditions were the following: (i) initial cell concentration of approximately 1.0 × 106 cells per mL, which corresponds to a biomass (cell dry weight – dw) concentration of about 0.05–0.08 gdw L−1; (ii) initial pH was set at 7; (iii) continuous aeration with the injection of atmospheric air (filtered through 0.22 μm cellulose acetate membranes, Orange Scientific, Belgium) at the bottom of the flasks. The assays were carried out under different temperatures (15, 25 and 35 °C) and incident light irradiances (36 and 180 μE m−2 s−1). The temperatures of 15, 25 and 35 °C were selected to simulate average temperatures observed in cold, warm and tropical regions, respectively. Light irradiance values were selected to observe the effect of low and high irradiance levels. Selection of this specific range of light irradiance values has taken into account the possible values that can be achieved using artificial light. For each temperature and irradiance value, different light cycles were evaluated: 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14, 14
14, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and 24
10, and 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (light
0 (light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark ratio). The light
dark ratio). The light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark ratio of 24
dark ratio of 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 was used because it promotes continuous photoautotrophic growth. To reduce production costs in terms of light requirements, the light
0 was used because it promotes continuous photoautotrophic growth. To reduce production costs in terms of light requirements, the light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark ratios of 10
dark ratios of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 and 14
14 and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 were applied to simulate the number of light hours during winter and summer time, respectively. For each studied condition, two independent experiments were performed. Taking into account the light irradiances and light
10 were applied to simulate the number of light hours during winter and summer time, respectively. For each studied condition, two independent experiments were performed. Taking into account the light irradiances and light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark ratios evaluated in this study, the corresponding average daily light irradiances are presented in Table 1.
dark ratios evaluated in this study, the corresponding average daily light irradiances are presented in Table 1.
Table 1 Average daily light irradiances evaluated in this study considering light irradiance and light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark ratio values applied to the selected cultures
dark ratio values applied to the selected cultures
| Light irradiance (μE m−2 s−1) |
Light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark ratio (h dark ratio (h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h) h) |
Average daily light irradiance (μE m−2 s−1) |
| 36 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
15 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
21 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
36 |
| 180 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
75 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
105 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
180 |
2.3. Growth monitoring and kinetic growth parameters
Duplicate samples were collected at 24 h intervals and biomass concentration was determined by measuring optical density at 750 nm, OD750,26 using a V-1200 spectrophotometer (VWR, Portugal). The relationship between OD750 and biomass concentration (X, mgdw L−1) for all microorganisms was established by linear regression, using the previously determined expressions.27 Biomass concentration values were used to determine specific growth rates (μ, d−1) and biomass productivities (P, mgdw L−1 d−1). Specific growth rates were determined according to eqn (1):28| |
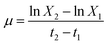 | (1) |
where X2 and X1 correspond to biomass concentration (in mgdw L−1) at times t2 and t1 (in days), the end and beginning of the exponential growth phase, respectively. Biomass productivities achieved in the exponential growth phase were calculated from the variation in biomass concentration within the exponential growth phase, as shown in eqn (2):28,29| |
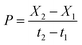 | (2) |
2.4. Nutrients removal
Nutrients removal was determined by quantification of nitrogen and phosphorus in the culture medium. For each analytical assay, one-millilitre samples from each culture were collected in the first and last day of culturing. Samples were centrifuged at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500g for 10 min and supernatants were stored at −20 °C until being analysed. Nitrate concentration was determined through UV spectroscopy at 220 nm using a T80 UV/VIS Spectrophotometer (PG Instruments, UK), according to the method proposed by Collos et al.30 On the other hand, inorganic phosphate quantification was performed by measuring absorbance at 820 nm of a phosphomolybdate complex formed by reaction of inorganic phosphate with ammonium molybdate in a Synergy™ HT 96-well microplate reader (Biotek Instruments, Inc., USA), as proposed by Lee et al.31 Nutrients concentration in the first and last day of culturing were used to determine average removal rates (RR, in mgS L−1 d−1) and nutrients removal efficiencies (R, in %). Average removal rates were calculated as follows:32
500g for 10 min and supernatants were stored at −20 °C until being analysed. Nitrate concentration was determined through UV spectroscopy at 220 nm using a T80 UV/VIS Spectrophotometer (PG Instruments, UK), according to the method proposed by Collos et al.30 On the other hand, inorganic phosphate quantification was performed by measuring absorbance at 820 nm of a phosphomolybdate complex formed by reaction of inorganic phosphate with ammonium molybdate in a Synergy™ HT 96-well microplate reader (Biotek Instruments, Inc., USA), as proposed by Lee et al.31 Nutrients concentration in the first and last day of culturing were used to determine average removal rates (RR, in mgS L−1 d−1) and nutrients removal efficiencies (R, in %). Average removal rates were calculated as follows:32| |
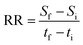 | (3) |
where Sf and Si correspond to nutrients concentration (in mgS L−1) at times tf and ti (in days), the end and beginning of cultivation time, respectively. Nutrients removal efficiencies were determined according to eqn (4):| |
 | (4) |
Additionally, for each nutrient a mass balance was written and the mass fractions (α, in gS gdw−1) of nitrogen and phosphorus incorporated in microalgal/cyanobacterial biomass were determined. This mass balance was determined according to eqn (5):33
| |
 | (5) |
where
S corresponds to nutrients concentration (in g
S L
−1). By integrating
eqn (5) over the cultivation time,
eqn (6) was obtained:
| | |
(Si − Sf) = α(Xf − Xi)
| (6) |
2.5. Modelling of microalgal growth
To determine the optimal growth conditions (average daily light irradiance and temperature) for the selected microalgae and cyanobacteria, a kinetic growth model was developed. Development of this model was based on specific growth rates determined for each of the studied microorganisms when grown under different light and temperature conditions. These data were obtained in this study and in other studies reported in the literature, as it is possible to see in Table S1 from the ESI.†
The behaviour of specific growth rates for increasing average daily light irradiance values was described according to the model proposed by Steele:20
| |
 | (7) |
where
μmax corresponds to the maximum specific growth rate (in d
−1) achieved by the studied microorganisms,
I denotes average daily light irradiance (in μE m
−2 s
−1) and
Iopt corresponds to the optimal value of average daily light irradiance (in μE m
−2 s
−1) for microalgal/cyanobacterial growth.
On the other hand, the behaviour of specific growth rates for different temperatures was assumed to follow a skewed normal distribution, as reported by Dauta et al.:34
| |
 | (8) |
where
T is the temperature (in °C),
Topt is the optimal temperature (in °C) for microalgal/cyanobacterial growth and
σ is the standard deviation associated to the optimal temperature (in °C).
Eqn (7) and (8) were used to establish a two-dimensional model, resulting in the following expression:
| |
 | (9) |
This expression was linearized (eqn (10)) and the parameters μmax, Iopt, Topt and σ were determined by minimizing the sum of squared residuals using the Solver supplement of Microsoft Excel 2013.
| |
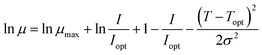 | (10) |
The quality of the model fits was evaluated by calculating the root mean squared error (RMSE), a performance index that measures the agreement between data obtained experimentally and predicted values:
| |
 | (11) |
where
z denotes the experimental values,
ẑ the predicted values by the model and
n the data size.
2.6. Statistical analysis
For each parameter, the average and standard deviation were calculated. The statistical significance of the results was evaluated using the Student's paired t-test to investigate whether the differences between the studied cultures could be considered significant. This analysis was performed using the statistical software SPSS 22.0 (SPSS Inc., Chicago, IL, USA). Statistical tests were carried out at a significance level of 0.05.
3. Results and discussion
3.1. Influence of light supply and temperature on microalgal/cyanobacterial growth
When growing autotrophically, microalgae and cyanobacteria strongly depend on light supply and temperature.8,9 These environmental factors influence growth dynamics (Fig. S1, ESI†), including the specific growth rates and biomass productivities, and also nutrients uptake from the culture medium. Fig. 1 shows the effect of average daily light irradiance and temperature on specific growth rates of the microalgae C. vulgaris and P. subcapitata (A and B) and the cyanobacteria S. salina and M. aeruginosa (C and D). Maximum biomass concentrations and biomass productivities achieved in the exponential growth phase under these conditions are shown in Table 2. Specific growth rates determined for the studied microorganisms ranged from 0.0188 ± 0.0033 d−1 (for P. subcapitata grown at 35 °C with an average daily light irradiance of 15 μE m−2 s−1) to 1.19 ± 0.04 d−1 (for C. vulgaris grown at 25 °C with an average daily light irradiance of 180 μE m−2 s−1). Regarding light supply, an increase in average daily light irradiance resulted in statistically higher (p < 0.05) specific growth rates. Several studies have already reported the increase of specific growth rates with increasing light supplies.12,35,36 A positive relationship between specific growth rates and average daily light irradiance is not surprising, since microalgal/cyanobacterial growth is mainly autotrophic, requiring light as the major energy source. These results indicate that higher light supplies favoured the photosynthetic activity of the studied microorganisms, which was confirmed by the increase observed in average pH of the studied cultures: from 8.12 ± 0.29 (at 15 μE m−2 s−1) to 8.76 ± 1.03 (at 180 μE m−2 s−1). The increase in pH of the culture medium is related to an increase in carbon uptake by microalgae or cyanobacteria and, hence, in photosynthetic activity.37 Culturing temperature also contributed to considerable changes in the specific growth rates of the studied microorganisms. Specific growth rates determined at 25 °C were statistically higher than those determined at 15 (p < 0.001) and 35 °C (p = 0.001). However, no statistical differences (p = 0.087) were observed between specific growth rates determined at 15 and 35 °C. These results indicate that the growth of the studied microorganisms in response to different temperatures may follow a normal distribution function, being the optimal culturing temperature approximately 25 °C. Evidence that the optimal temperature for autotrophic microalgal/cyanobacterial growth is near 25 °C was also given by the increase observed in pH and dissolved oxygen concentration at this temperature: for cultures performed at 15, 25 and 35 °C average pH of the culture medium was 8.32 ± 0.43, 8.91 ± 0.91 and 8.09 ± 0.82, respectively, whereas average dissolved oxygen concentration was 3.8 ± 1.1, 6.5 ± 0.4 and 4.8 ± 1.0 mgO2 L−1, respectively. A similar behaviour was observed by James et al.38 when evaluating the effect of temperature on the growth and fatty acid and amino acid composition of two microalgae belonging to the genera Chlorella and Nannochloropsis. For temperatures ranging from 15 to 35 °C, an increase in specific growth rates was observed until 25 °C, while for higher temperatures specific growth rates started decreasing. Similarly, when evaluating the optimum temperature and salinity conditions for the growth of Chlorella ellipsoidea and Nannochloris oculata, Cho et al.39 demonstrated that keeping a constant salinity of 10, an increase in temperatures from 15 to 25 °C results in increased specific growth rates and, when temperature is increased to 30 °C, specific growth rates tend to decrease. Average specific growth rates determined for Chlorella pyrenoidosa grown under a temperature range of 10 to 35 °C also increased until the temperature of 25 °C, starting decreasing when culturing temperature was set at 30 and 35 °C.40
 |
| | Fig. 1 Specific growth rates, in d−1, determined for C. vulgaris (A), P. subcapitata (B), S. salina (C) and M. aeruginosa (D) under different light and temperature conditions. Error bars correspond to the standard deviation of two independent experiments. | |
Table 2 Maximum biomass concentrations (Xmax, in mgdw L−1) and biomass productivities achieved in the exponential growth phase (P, in mgdw L−1 d−1) determined for C. vulgaris, P. subcapitata, S. salina and M. aeruginosa grown under different light and temperature conditionsa
| Temperature (°C) |
Average daily light irradiance (μE m−2 s−1) |
C. vulgaris |
P. subcapitata |
S. salina |
M. aeruginosa |
| Xmax (mgdw L−1) |
P (mgdw L−1 d−1) |
Xmax (mgdw L−1) |
P (mgdw L−1 d−1) |
Xmax (mgdw L−1) |
P (mgdw L−1 d−1) |
Xmax (mgdw L−1) |
P (mgdw L−1 d−1) |
| Values are presented as the mean ± standard deviation of two independent experiments. |
| 15 |
15 |
73.9 ± 4.5 |
6.91 ± 2.46 |
49.7 ± 13.1 |
3.60 ± 0.40 |
167 ± 1 |
4.66 ± 1.55 |
72.6 ± 1.0 |
7.85 ± 2.34 |
| 21 |
107 ± 19 |
6.40 ± 4.24 |
70.8 ± 4 |
10.8 ± 3.4 |
173 ± 12 |
10.4 ± 1.2 |
109 ± 20 |
10.2 ± 1.5 |
| 36 |
194 ± 52 |
17.5 ± 1.6 |
107 ± 25 |
10.1 ± 2.1 |
242 ± 13 |
5.12 ± 1.36 |
189 ± 29 |
5.37 ± 0.94 |
| 75 |
331 ± 46 |
12.9 ± 1.0 |
113 ± 3 |
11.2 ± 0.9 |
349 ± 11 |
6.49 ± 0.58 |
211 ± 11 |
12.6 ± 3.3 |
| 105 |
293 ± 20 |
15.4 ± 2.6 |
134 ± 5 |
23.4 ± 2.2 |
363 ± 20 |
6.03 ± 2.67 |
290 ± 7 |
10.2 ± 1.9 |
| 180 |
588 ± 71 |
23.2 ± 0.4 |
459 ± 27 |
41.4 ± 1.9 |
501 ± 33 |
33.7 ± 0.6 |
458 ± 7 |
26.0 ± 1.5 |
| 25 |
15 |
414 ± 13 |
13.5 ± 0.3 |
234 ± 25 |
8.43 ± 0.94 |
426 ± 24 |
9.25 ± 1.39 |
406 ± 16 |
22.8 ± 1.3 |
| 21 |
517 ± 11 |
29.4 ± 2.2 |
249 ± 13 |
16.5 ± 2.3 |
481 ± 19 |
17.4 ± 3.1 |
484 ± 7 |
30.4 ± 1.9 |
| 36 |
828 ± 23 |
49.7 ± 3.9 |
426 ± 15 |
33.9 ± 0.7 |
738 ± 16 |
36.2 ± 1.4 |
742 ± 3 |
44.3 ± 2.8 |
| 75 |
771 ± 11 |
31.7 ± 2.5 |
488 ± 13 |
32.6 ± 0.8 |
719 ± 39 |
27.9 ± 6.2 |
767 ± 17 |
40.8 ± 2.4 |
| 105 |
(1.08 ± 0.14) × 103 |
95.5 ± 9.5 |
697 ± 7 |
82.4 ± 7.8 |
914 ± 30 |
78.0 ± 6.4 |
991 ± 7 |
97.4 ± 6.3 |
| 180 |
(1.35 ± 0.13) × 103 |
125 ± 8 |
798 ± 36 |
110 ± 6 |
(1.26 ± 0.06) × 103 |
111 ± 6 |
(1.17 ± 0.06) × 103 |
120 ± 16 |
| 35 |
15 |
93.4 ± 6.5 |
4.57 ± 0.24 |
3.94 ± 0.49 |
0.206 ± 0.111 |
172 ± 1 |
6.49 ± 0.58 |
71.7 ± 2.5 |
9.08 ± 0.53 |
| 21 |
108 ± 2 |
5.16 ± 0.70 |
12.7 ± 1.1 |
0.418 ± 0.232 |
228 ± 16 |
13.4 ± 3.3 |
131 ± 17 |
12.6 ± 3.3 |
| 36 |
152 ± 10 |
13.4 ± 0.8 |
15.9 ± 2.5 |
2.32 ± 1.23 |
260 ± 25 |
17.0 ± 3.7 |
177 ± 8 |
16.8 ± 3.7 |
| 75 |
396 ± 29 |
31.8 ± 1.0 |
190 ± 5 |
22.2 ± 2.0 |
309 ± 7 |
26.5 ± 2.0 |
220 ± 26 |
17.4 ± 2.7 |
| 105 |
527 ± 28 |
50.1 ± 0.9 |
366 ± 24 |
31.6 ± 4.2 |
461 ± 12 |
30.4 ± 4.1 |
391 ± 7 |
40.4 ± 6.3 |
| 180 |
518 ± 58 |
48.7 ± 7.9 |
290 ± 19 |
30.2 ± 0.7 |
436 ± 20 |
38.2 ± 3.8 |
371 ± 26 |
39.8 ± 11.4 |
The influence of light supply and temperature on maximum biomass concentrations and biomass productivities was similar to the one observed for specific growth rates (Table 2). In this study maximum biomass concentration values ranged from 3.94 ± 0.49 (determined for P. subcapitata grown at 35 °C with an average daily light irradiance of 15 μE m−2 s−1) to (1.35 ± 0.13) × 103 mgdw L−1 (determined for C. vulgaris grown at 25 °C with an average daily light irradiance of 180 μE m−2 s−1). Minimum and maximum biomass productivities were determined for the same microorganisms in the same conditions: 0.206 ± 0.111 (for P. subcapitata grown at 35 °C with an average daily light irradiance of 15 μE m−2 s−1) and 125 ± 8 mgdw L−1 d−1 (for C. vulgaris grown at 25 °C with an average daily light irradiance of 180 μE m−2 s−1), respectively. As for specific growth rates, an increase in average daily light irradiance from 15 to 180 μE m−2 s−1 resulted in statistically higher (p < 0.05) maximum biomass concentrations and biomass productivities. Ugwu et al.41 demonstrated that an increase in light irradiance results in an increase in biomass productivities when growing Chlorella sorokiniana with average daily light irradiances ranging from 100 to 250 μE m−2 s−1. Regarding the effects of temperature, statistically higher (p < 0.05) maximum biomass concentrations and biomass productivities were determined for cultures grown at 25 °C. In the case of cultures grown at 15 and 35 °C, no statistical difference (p > 0.05) was observed in both maximum biomass concentrations and biomass productivities. Han et al.42 found that cultivation of C. pyrenoidosa at 22, 30 and 36 °C resulted in biomass productivities of 120 ± 2, 141 ± 1 and 125 ± 2 mg L−1 d−1, respectively.
Comparing kinetic growth parameters determined for the studied microorganisms, it was possible to observe that C. vulgaris achieved the highest specific growth rate, maximum biomass concentration and biomass productivity when cultured at 25 °C under an average daily light irradiance of 180 μE m−2 s−1. In the same culturing conditions specific growth rates determined for P. subcapitata and S. salina were not statistically different (p > 0.05) from the one determined for C. vulgaris. In the case of M. aeruginosa, specific growth rate determined in these conditions was statistically lower (p < 0.05). Regarding maximum biomass concentrations and biomass productivities, values determined for S. salina and M. aeruginosa were not statistically different (p > 0.05) from those determined for C. vulgaris. However, statistically lower (p < 0.05) values were determined for P. subcapitata.
3.2. Influence of light supply and temperature on nutrients removal
To evaluate the influence of light supply and temperature on nitrogen and phosphorus removal, concentrations of these nutrients in the first and last day of culturing were determined and average removal rates and removal efficiencies were obtained. These results are shown in Table 3, for nitrogen, and Table 4, for phosphorus.
Table 3 Average nitrogen removal rates (RR, in mgN L−1 d−1) and nitrogen removal efficiencies (R, in %) determined for C. vulgaris, P. subcapitata, S. salina and M. aeruginosa grown under different light and temperature conditionsa
| Temperature (°C) |
Average daily light irradiance (μE m−2 s−1) |
C. vulgaris |
P. subcapitata |
S. salina |
M. aeruginosa |
| RR (mgN L−1 d−1) |
R (%) |
RR (mgN L−1 d−1) |
R (%) |
RR (mgN L−1 d−1) |
R (%) |
RR (mgN L−1 d−1) |
R (%) |
| Values are presented as the mean ± standard deviation of two independent experiments. |
| 15 |
15 |
0.658 ± 0.277 |
36.8 ± 9.6 |
0.115 ± 0.061 |
7.55 ± 3.62 |
0.278 ± 0.199 |
8.98 ± 6.55 |
0.497 ± 0.151 |
16.5 ± 4.7 |
| 21 |
0.561 ± 0.035 |
37.9 ± 1.7 |
0.221 ± 0.098 |
16.5 ± 7.1 |
0.723 ± 0.161 |
25.3 ± 6.0 |
0.827 ± 0.250 |
27.1 ± 5.8 |
| 36 |
1.67 ± 0.69 |
78.9 ± 6.0 |
0.472 ± 0.100 |
28.3 ± 5.8 |
0.816 ± 0.141 |
30.0 ± 5.8 |
1.21 ± 0.15 |
40.2 ± 4.9 |
| 75 |
0.759 ± 0.225 |
24.8 ± 9.0 |
0.713 ± 0.474 |
25.3 ± 13.2 |
1.45 ± 0.33 |
45.7 ± 13.8 |
1.17 ± 0.12 |
41.1 ± 3.2 |
| 105 |
2.11 ± 0.07 |
77.2 ± 5.6 |
1.69 ± 0.54 |
50.5 ± 10.0 |
2.32 ± 0.31 |
68.3 ± 5.0 |
1.87 ± 0.28 |
69.8 ± 3.3 |
| 180 |
2.56 ± 0.49 |
93.4 ± 9.8 |
2.36 ± 0.25 |
79.1 ± 4.2 |
2.33 ± 0.27 |
75.0 ± 13.1 |
2.58 ± 0.34 |
85.3 ± 6.3 |
| 25 |
15 |
1.08 ± 0.03 |
42.3 ± 1.6 |
1.07 ± 0.21 |
43.5 ± 8.3 |
1.27 ± 0.02 |
48.5 ± 0.7 |
1.42 ± 0.04 |
53.6 ± 1.7 |
| 21 |
1.69 ± 0.16 |
75.6 ± 5.8 |
1.24 ± 0.04 |
74.4 ± 2.9 |
1.86 ± 0.06 |
96.1 ± 0.9 |
1.82 ± 0.03 |
98.8 ± 1.4 |
| 36 |
2.43 ± 0.38 |
97.1 ± 1.7 |
2.62 ± 0.08 |
88.0 ± 2.7 |
2.83 ± 0.16 |
92.5 ± 1.0 |
2.89 ± 0.07 |
97.3 ± 1.1 |
| 75 |
2.40 ± 0.05 |
86.2 ± 1.7 |
1.97 ± 0.02 |
68.9 ± 0.8 |
2.45 ± 0.02 |
86.1 ± 0.6 |
2.59 ± 0.03 |
89.8 ± 0.4 |
| 105 |
2.78 ± 0.06 |
98.0 ± 2.0 |
2.16 ± 0.54 |
97.7 ± 2.5 |
2.54 ± 0.20 |
98.6 ± 0.4 |
2.43 ± 0.33 |
98.0 ± 0.6 |
| 180 |
2.43 ± 0.40 |
100 ± 0 |
2.37 ± 0.18 |
100 ± 0 |
1.97 ± 0.19 |
99.1 ± 0.7 |
2.53 ± 0.21 |
100 ± 0 |
| 35 |
15 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| 21 |
0.131 ± 0.039 |
6.68 ± 1.93 |
0 |
0 |
0.0836 ± 0.0091 |
0 |
0.0115 ± 0.0006 |
0.0510 ± 0.0141 |
| 36 |
0.482 ± 0.292 |
16.3 ± 8.2 |
0.0442 ± 0.0071 |
1.37 ± 0.75 |
0.330 ± 0.081 |
15.1 ± 3.0 |
0.0874 ± 0.0360 |
4.00 ± 1.55 |
| 75 |
0.959 ± 0.558 |
37.0 ± 21.3 |
0.804 ± 0.246 |
30.9 ± 9.2 |
2.22 ± 0.87 |
58.7 ± 9.5 |
1.47 ± 0.11 |
53.5 ± 2.4 |
| 105 |
1.60 ± 0.12 |
63.4 ± 4.8 |
1.75 ± 0.07 |
70.6 ± 2.7 |
1.29 ± 0.01 |
61.4 ± 0.6 |
1.85 ± 0.06 |
73.5 ± 1.6 |
| 180 |
2.41 ± 0.04 |
88.6 ± 1.5 |
1.95 ± 0.05 |
78.1 ± 1.6 |
1.25 ± 0.12 |
63.8 ± 1.9 |
2.14 ± 0.02 |
91.1 ± 0.6 |
Table 4 Average phosphorus removal rates (RR, in mgP L−1 d−1) and phosphorus removal efficiencies (R, in %) determined for C. vulgaris, P. subcapitata, S. salina and M. aeruginosa grown under different light and temperature conditionsa
| Temperature (°C) |
Average daily light irradiance (μE m−2 s−1) |
C. vulgaris |
P. subcapitata |
S. salina |
M. aeruginosa |
| RR (mgP L−1 d−1) |
R (%) |
RR (mgP L−1 d−1) |
R (%) |
RR (mgP L−1 d−1) |
R (%) |
RR (mgP L−1 d−1) |
R (%) |
| Values are presented as the mean ± standard deviation of two independent experiments. |
| 15 |
15 |
0.110 ± 0.013 |
13.5 ± 1.6 |
0.0505 ± 0.0154 |
6.18 ± 1.74 |
0.0171 ± 0.0092 |
1.97 ± 1.09 |
0.00944 ± 0.00035 |
1.13 ± 0.03 |
| 21 |
0.0934 ± 0.0607 |
11.8 ± 7.2 |
0.220 ± 0.044 |
26.2 ± 4.3 |
0.107 ± 0.026 |
10.9 ± 2.5 |
0.120 ± 0.060 |
12.4 ± 5.7 |
| 36 |
0.265 ± 0.037 |
32.7 ± 4.5 |
0.158 ± 0.087 |
20.6 ± 12.2 |
0.126 ± 0.047 |
13.4 ± 5.1 |
0.182 ± 0.067 |
18.3 ± 6.2 |
| 75 |
0.275 ± 0.025 |
29.5 ± 3.0 |
0.0751 ± 0.0061 |
9.47 ± 0.67 |
0.386 ± 0.089 |
44.6 ± 9.5 |
0.416 ± 0.031 |
26.3 ± 2.1 |
| 105 |
0.255 ± 0.130 |
29.1 ± 12.3 |
0.157 ± 0.068 |
20.1 ± 9.7 |
0.215 ± 0.034 |
20.9 ± 4.4 |
0.389 ± 0.050 |
37.8 ± 0.9 |
| 180 |
0.387 ± 0.010 |
44.2 ± 1.0 |
0.252 ± 0.073 |
27.5 ± 6.0 |
0.275 ± 0.008 |
29.1 ± 1.0 |
0.255 ± 0.027 |
21.4 ± 3.1 |
| 25 |
15 |
0.149 ± 0.035 |
16.9 ± 3.4 |
0.268 ± 0.115 |
17.5 ± 7.9 |
0.157 ± 0.007 |
17.3 ± 0.6 |
0.109 ± 0.081 |
13.4 ± 8.8 |
| 21 |
0.258 ± 0.019 |
29.3 ± 1.6 |
0.223 ± 0.057 |
24.0 ± 9.6 |
0.222 ± 0.034 |
23.9 ± 3.0 |
0.279 ± 0.081 |
28.8 ± 6.6 |
| 36 |
0.279 ± 0.092 |
29.3 ± 7.4 |
0.259 ± 0.056 |
34.2 ± 4.9 |
0.316 ± 0.034 |
35.4 ± 3.4 |
0.255 ± 0.068 |
29.7 ± 6.0 |
| 75 |
0.240 ± 0.191 |
24.9 ± 18.4 |
0.235 ± 0.018 |
27.0 ± 2.0 |
0.231 ± 0.064 |
33.9 ± 0.6 |
0.218 ± 0.050 |
26.3 ± 5.7 |
| 105 |
0.240 ± 0.074 |
31.5 ± 4.0 |
0.279 ± 0.020 |
32.7 ± 2.0 |
0.345 ± 0.035 |
32.0 ± 4.8 |
0.231 ± 0.039 |
25.8 ± 2.1 |
| 180 |
0.588 ± 0.029 |
67.6 ± 7.1 |
0.393 ± 0.070 |
51.2 ± 4.8 |
0.348 ± 0.018 |
36.7 ± 4.3 |
0.357 ± 0.074 |
41.1 ± 9.2 |
| 35 |
15 |
0.0767 ± 0.0300 |
7.76 ± 2.60 |
0.0785 ± 0.0109 |
7.89 ± 0.67 |
0.0642 ± 0.0495 |
6.67 ± 4.98 |
0.063 ± 0.049 |
6.56 ± 4.90 |
| 21 |
0.160 ± 0.017 |
16.4 ± 3.0 |
0.143 ± 0.026 |
14.6 ± 3.5 |
0.167 ± 0.029 |
16.8 ± 4.1 |
0.137 ± 0.027 |
13.1 ± 3.4 |
| 36 |
0.171 ± 0.047 |
16.8 ± 3.9 |
0.184 ± 0.070 |
17.5 ± 5.6 |
0.188 ± 0.066 |
17.9 ± 5.4 |
0.157 ± 0.060 |
15.0 ± 5.1 |
| 75 |
0.895 ± 0.015 |
21.0 ± 1.7 |
0.0968 ± 0.0213 |
9.84 ± 2.07 |
0.378 ± 0.006 |
42.9 ± 0.8 |
0.282 ± 0.030 |
26.1 ± 2.5 |
| 105 |
0.316 ± 0.021 |
33.3 ± 2.0 |
0.241 ± 0.020 |
26.6 ± 2.2 |
0.194 ± 0.036 |
21.0 ± 4.6 |
0.352 ± 0.027 |
36.0 ± 2.5 |
| 180 |
0.278 ± 0.063 |
38.3 ± 14.1 |
0.440 ± 0.067 |
38.7 ± 4.3 |
0.210 ± 0.046 |
22.7 ± 4.3 |
0.543 ± 0.072 |
54.2 ± 3.2 |
Regarding nitrogen removal, maximum average removal rate, 2.89 ± 0.07 mgN L−1 d−1, was determined for M. aeruginosa grown at 25 °C, with an average daily light irradiance of 36 μE m−2 s−1. On the other hand, maximum nitrogen removal efficiency achieved was 100% (for C. vulgaris, P. subcapitata and M. aeruginosa grown at 25 °C with an average daily light irradiance of 180 μE m−2 s−1). The influence of light supply and temperature in these variables was very similar. In the case of average daily light irradiance, higher values resulted in statistically higher (p < 0.05) removal rates and removal efficiencies. In the study performed by Hu et al.,43 nitrate uptake rates determined for Synechococcus sp. grown in nitrate-contaminated groundwater increased proportionally to increasing average daily light irradiance up to 100 μE m−2 s−1. Regarding the effects of temperature, microalgal and cyanobacterial growth at 25 °C caused nitrogen removal rates and removal efficiencies statistically higher (p < 0.05) than those determined at 15 and 35 °C. The nitrogen removal rates and removal efficiencies were not statistically different (p = 0.146) between the extreme temperatures. Talbot and De la Noüe44 demonstrated that cultivation of Phormidium bohneri in a secondary effluent from an activated sludge treatment plant at 30 °C for three days resulted in an effective removal of ammonia-nitrogen, whereas the same culture performed at 10 °C resulted in modest ammonia-nitrogen removal.
In the case of phosphorus removal, maximum average removal rate, 0.588 ± 0.029 mgP L−1 d−1, was determined for C. vulgaris grown at 25 °C with an average daily light irradiance of 180 μE m−2 s−1. Phosphorus removal efficiencies ranged from 1.13 ± 0.03 (for M. aeruginosa grown at 15 °C, under the lowest average daily light irradiance) to 67.6 ± 7.1% (for C. vulgaris grown at 25 °C with an average daily light irradiance of 180 μE m−2 s−1). These values were lower than those determined for nitrate, indicating that phosphorus assimilation is slower than nitrate-nitrogen assimilation. Different studies have already reported higher removal efficiencies for nitrogen than for phosphorus.44,45 The influence of light supply and temperature on phosphorus removal rates and removal efficiencies was similar to the one observed for nitrogen removal. In general, an increase in the light supply resulted in increased phosphorus removal rates and removal efficiencies. Statistically higher (p < 0.05) removal rates and removal efficiencies were determined when light irradiance increased from 15 to 180 μE m−2 s−1. In the study performed by Li et al.,46 an increase in average daily light irradiance from 0 to 200 μE m−2 s−1 increased total phosphorus removal efficiencies from 65.8 to 87.0% (for Chlorella kessleri) and from 79.3 to 83.0% (for Chlorella protothecoides). The effects of temperature on phosphorus removal demonstrated that, in general, higher removal rates and removal efficiencies were obtained for cultures grown at 25 °C. However, these values were not statistically different (p > 0.05) from those determined for the other studied temperatures.
These results have shown that the influence of light supply and temperature on nitrogen and phosphorus removal is similar to the one observed for specific growth rates, maximum biomass concentrations and biomass productivities, paralleling photosynthetic activity. Microalgae and cyanobacteria require high amounts of nitrogen and phosphorus for proteins, which account for 40–60% of cell dry weight, nucleic acids and phospholipids synthesis,3 meaning that an increase in the photosynthetic activity may result in an increased assimilation of both nitrogen and phosphorus. Regarding the performance of the studied microorganisms in nitrogen and phosphorus removal, average removal rates and removal efficiencies were not statistically different (p > 0.05). Additionally, it was observed that the majority of cultures grown at 25 °C, under the highest light supplies have effectively removed nitrogen. These results constitute important findings for the application of microalgal/cyanobacterial cultures in the tertiary treatment step of wastewater treatment plants.
The mass balance written for nitrogen and phosphorus allowed the determination of the mass fractions of these nutrients in the biomass for each of the studied conditions (Table 5). Mass fractions of nitrogen and phosphorus were close to those reported in the typical composition of microalgal biomass (CO0.48H1.83N0.11P0.01): 6.59 gN gdw−1 and 1.33 gP gdw−1 for nitrogen and phosphorus, respectively.47 To have a better understanding about the effects of light and temperature on nitrogen and phosphorus contents on microalgal/cyanobacterial biomass, contour graphs relating these variables were obtained for the selected microorganisms (Fig. S2 and S3, ESI†). Additionally, these parameters were analysed through multiple linear regression to evaluate which parameters significantly influence nitrogen and phosphorus mass fractions (Table S2, ESI†). From these data, it is possible to conclude that the effect of light and temperature on the biochemical composition of microalgal/cyanobacterial biomass presented some differences between the studied microorganisms. These observations are in agreement with the study performed by Goldman,48 who concluded that the relationship between nitrogen contents and temperature may be species specific. Regarding nitrogen mass fractions, temperature appears as the most important factor influencing this parameter: (i) in the case of C. vulgaris and S. salina, an increase in temperature results in lower nitrogen mass fractions; (ii) in P. subcapitata, both light and temperature have not significantly influenced (p > 0.05) nitrogen mass fractions; and (iii) in M. aeruginosa, an increase in light and temperature results in lower nitrogen mass fractions and, on the other hand, the simultaneous increase in both light and temperature results in higher nitrogen mass fractions. As for nitrogen mass fractions, phosphorus mass fractions were also mainly influenced by temperature: (i) in C. vulgaris, an increase in temperature results in a decrease of phosphorus mass fractions, with the minimum value reached at approximately 25 °C, and the simultaneous increase in both light and temperature results in lower phosphorus mass fractions; (ii) in P. subcapitata, phosphorus mass fractions had a similar behaviour to the one described for nitrogen mass fractions in M. aeruginosa; and (iii) in S. salina and M. aeruginosa, an increase in temperature results in a decrease of phosphorus mass fractions, with the minimum value reached at approximately 25 °C. These results indicate that environmental factors, such as light and temperature, not only affect the photosynthetic activity and biomass productivities, but also cell metabolism and, consequently, biochemical composition, as previously reported by Hu.9 The preponderance of temperature influence on nitrogen and phosphorus mass fractions behaviour suggests that these parameters were not strongly influenced by average daily light irradiance. Similar results were already reported by Mortensen et al.49 In this study, nitrogen and phosphorus mass fractions determined for batch cultures of Chaetoceros gracilis grown with different light intensities at 28 °C were not statistically different. The decrease of nitrogen and phosphorus mass fractions with increasing temperatures, which was common for the majority of the selected microorganisms has already been reported in the literature. In the study performed by Fu et al.50 an increase in temperature from 20 to 24 °C resulted in a decrease in nitrogen and phosphorus mass fractions in the cyanobacteria Synechococcus sp. The U-shape response observed for some microorganisms has also been described in the literature. According to Hu,9 at temperatures below and above the optimal growth temperature, microalgae and cyanobacteria require higher amounts of nutrients, such as nitrogen and phosphorus, to achieve the same growth rates as those reported for optimal temperatures. Accordingly, nitrogen and phosphorus mass fractions tend to be lower at the optimal growth temperature, which was, in this study, around 25 °C.
Table 5 Mass fractions of nitrogen (αN, in gN gdw−1) and phosphorus (αP, in gP gdw−1) incorporated in the biomass of C. vulgaris, P. subcapitata, S. salina and M. aeruginosa obtained through the mass balance performed for each nutrienta
| Temperature (°C) |
Average daily light irradiance (μE m−2 s−1) |
C. vulgaris |
P. subcapitata |
S. salina |
M. aeruginosa |
| αN (gN gdw−1) |
αP (gP gdw−1) |
αN (gN gdw−1) |
αP (gP gdw−1) |
αN (gN gdw−1) |
αP (gP gdw−1) |
αN (gN gdw−1) |
αP (gP gdw−1) |
| n.a. – not applicable. |
| 15 |
15 |
0.142 |
0.0239 |
0.0278 |
0.0122 |
0.0505 |
0.00311 |
0.0950 |
0.00181 |
| 21 |
0.0680 |
0.0113 |
0.0374 |
0.0372 |
0.116 |
0.0170 |
0.0941 |
0.0136 |
| 36 |
0.102 |
0.0161 |
0.0498 |
0.0166 |
0.0689 |
0.0106 |
0.0772 |
0.0116 |
| 75 |
0.0288 |
0.0105 |
0.0767 |
0.00807 |
0.0689 |
0.0184 |
0.0675 |
0.0240 |
| 105 |
0.0892 |
0.0108 |
0.146 |
0.0136 |
0.100 |
0.00927 |
0.0748 |
0.0156 |
| 180 |
0.0524 |
0.00793 |
0.0583 |
0.00623 |
0.0675 |
0.00797 |
0.0650 |
0.00643 |
| 25 |
15 |
0.0298 |
0.00412 |
0.0515 |
0.0129 |
0.0445 |
0.00548 |
0.0425 |
0.00326 |
| 21 |
0.0373 |
0.00570 |
0.0558 |
0.0100 |
0.0560 |
0.00669 |
0.0452 |
0.00692 |
| 36 |
0.0328 |
0.00377 |
0.0679 |
0.00672 |
0.0495 |
0.00552 |
0.0450 |
0.00397 |
| 75 |
0.0349 |
0.00348 |
0.0444 |
0.0053 |
0.0441 |
0.00416 |
0.0390 |
0.00329 |
| 105 |
0.0286 |
0.00248 |
0.0343 |
0.0044 |
0.0348 |
0.00473 |
0.0281 |
0.00266 |
| 180 |
0.0200 |
0.00485 |
0.0329 |
0.00545 |
0.0189 |
0.00334 |
0.0245 |
0.00345 |
| 35 |
15 |
n.a. |
0.0151 |
n.a. |
0.219 |
n.a. |
0.0130 |
n.a. |
0.0158 |
| 21 |
0.0192 |
0.0235 |
n.a. |
0.124 |
0.00856 |
0.0171 |
0.000127 |
0.0139 |
| 36 |
0.0452 |
0.0160 |
0.0660 |
0.275 |
0.0254 |
0.0145 |
0.00638 |
0.0115 |
| 75 |
0.0286 |
0.00607 |
0.0494 |
0.00595 |
0.132 |
0.0224 |
0.0866 |
0.0167 |
| 105 |
0.0343 |
0.00675 |
0.0534 |
0.00735 |
0.0420 |
0.00631 |
0.0214 |
0.00407 |
| 180 |
0.0526 |
0.00608 |
0.0747 |
0.0169 |
0.0422 |
0.00711 |
0.0689 |
0.0175 |
3.3. Optimal light and temperature conditions determined through mathematical modelling
Optimal growth conditions (average daily light irradiance and temperature) for the selected microalgae and cyanobacteria were determined. For this, the model described by eqn (9) was applied and surface graphs (Fig. 2) relating specific growth rates with average daily light irradiance and temperature were obtained. Analysis of Fig. 2 shows that an increase in average daily light irradiance results in increased specific growth rates, with optimal average daily light irradiances varying according to the studied species. Regarding the effect of temperature on specific growth rates, Fig. 2 evidences a similar behaviour between the studied microorganisms. When temperature increases from 15 to 35 °C, specific growth rates tend to increase until approximately 25 °C, where specific growth rates start decreasing, reaching values close to those observed at 15 °C.
 |
| | Fig. 2 Influence of average daily light irradiance and temperature on specific growth rates of C. vulgaris (A), P. subcapitata (B), S. salina (C) and M. aeruginosa (D). The dots correspond to the experimental data. The surface graphs were obtained through mathematical modelling. | |
Optimal average daily light irradiance and temperature determined through mathematical modelling for each microorganism are shown in Table 6. For determination of these parameters, it was assumed that maximum specific growth rates achieved by each microorganism could not be lower than the maximum specific growth rate value determined for each microalgal/cyanobacterial strain: 1.30, 1.13, 1.14 and 1.02 d−1 for C. vulgaris, P. subcapitata, S. salina and M. aeruginosa, respectively. Definition of this condition was based on the fact that each microalgal species usually presents a maximum specific growth rate, which is obtained under optimal growth conditions.51 From Table 6, it is possible to observe that optimal temperatures determined for the studied microorganisms were very similar. Topt values determined through mathematical modelling for C. vulgaris, P. subcapitata, S. salina and M. aeruginosa were 25.4, 23.7, 26.4 and 25.6 °C, respectively. These values were slightly lower than optimal temperature determined for C. vulgaris growth in the study performed by Dauta et al.34 In this study, for a maximum specific growth rate of 1.30 d−1, optimal temperature determined for C. vulgaris was 30 °C. However, other studies reported optimal growth temperatures close to 25 °C. In the study performed by Claquin et al.,52 average optimal temperature determined for eight species of marine microalgae (Thalassiosira pseudonana, Skeletonema marinoi, Pseudo-nitzschia fraudulenta, Emiliania huxleyi, Isochrysis galbana, Isochrysis aff. galbana, Pavlova lutheri and Lepidodinium chlorophorum) was 23.7 ± 3.1 °C, corresponding to a maximum specific growth rate of 1.27 ± 0.27 d−1. Yang et al.40 demonstrated that C. vulgaris can grow normally in the temperature range of 5 to 30 °C, being optimal growth temperature 25 °C. Through mathematical modelling, Aleya et al.53 determined an optimal growth temperature for Chlorella minutissima of 28 °C, corresponding to a maximum specific growth rate of 0.7 d−1. Regarding optimal average daily light irradiances determined using this model, Table 6 shows that Iopt values differ according to microalgal/cyanobacterial species, being 208, 258, 178 and 140 μE m−2 s−1 for C. vulgaris, P. subcapitata, S. salina and M. aeruginosa, respectively. Similar orders of magnitude have already been reported in the literature for several microalgae and cyanobacteria. Optimal average daily light irradiance values determined by Dauta et al.34 for C. vulgaris, Fragilaria crotonensis, Staurastrum pingue and Synechocystis minima ranged from 78 to 169 μE m−2 s−1. On the other hand, optimal average daily light irradiances determined for Selenastrum minutum, Coelastrum microporum f. astroidea and Cosmarium subprotumidum ranged from 250 to 263 μE m−2 s−1.51 However, optimal average daily light irradiance determined for C. vulgaris and P. subcapitata surpassed the range of values assessed in this study, meaning that optimal growth of these microalgae is expected to occur for an average daily light irradiance of 208 and 258 μE m−2 s−1, respectively. Although these results were not validated experimentally, it is possible to propose that the established models can be correctly applied to describe the response of specific growth rates of the studied microorganisms to light and temperature. In fact, optimal light and temperature conditions determined are in accordance with the ones already reported in the literature. Additionally, the low RMSE values determined (ranging from 0.198 to 0.319 d−1) indicate that these models correctly fit to the experimental data. Nevertheless, the current models were validated by evaluating the RMSE values obtained between specific growth rates determined by these models and a validation data set composed by specific growth rates determined in different light and temperature conditions (Table S3, ESI†). With the current models, RMSE values determined for C. vulgaris, P. subcapitata, S. salina and M. aeruginosa were 0.294, 0.198, 0.319 and 0.255 d−1, respectively. On the other hand, RMSE determined through application of this model to data obtained from other studies (validation data set) was 0.393, 0.283, 0.260 and 0.182 d−1, respectively. These results indicate that the developed model can be correctly applied to the studied microorganisms grown under light and temperature conditions within the range of those reported in this study. Additionally, in this study specific mathematical models were determined for different microalgal/cyanobacterial species. Determination of an adequate model that describes microalgal/cyanobacterial growth in relation to light supply and temperature may result in several savings, especially in the optimization of cultivation conditions.
Table 6 Optimal growth conditions (average daily light irradiance and temperature) determined for C. vulgaris, P. subcapitata, S. salina and M. aeruginosa through mathematical modellinga
| |
C. vulgaris |
P. subcapitata |
S. salina |
M. aeruginosa |
| These values were obtained through application of the developed model regarding the effect of light irradiance and temperature on specific growth rates. μmax – maximum specific growth rate; Iopt – optimal average daily light irradiance value for microalgal/cyanobacterial growth; Topt – optimal temperature for microalgal/cyanobacterial growth; σ – standard deviation associated to the optimal temperature; RMSE – root mean squared error; n – data size. |
| μmax (d−1) |
1.30 |
1.21 |
1.14 |
1.02 |
| Iopt (μE m−2 s−1) |
208 |
258 |
178 |
140 |
| Topt (°C) |
25.4 |
23.7 |
26.4 |
25.6 |
| σ (°C) |
7.0 |
7.0 |
7.2 |
8.2 |
| RMSE (d−1) |
0.294 |
0.198 |
0.319 |
0.255 |
| n |
29 |
27 |
18 |
18 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| Model validation |
| RMSE (d−1) |
0.393 |
0.283 |
0.260 |
0.182 |
| n |
9 |
9 |
6 |
6 |
4. Conclusions
In this study, the effects of average daily light irradiance and temperature on microalgal/cyanobacterial growth and nutrients (nitrogen and phosphorus) uptake was evaluated. The results have shown that increased light supplies favour both biomass productivities and nutrients removal. Regarding the temperature effect, it was observed that the studied microorganisms presented higher photosynthetic activity at 25 °C. Among the studied microorganisms, C. vulgaris, S. salina and M. aeruginosa have shown to be the most effective in biomass production. Development of a mathematical model able to describe the behaviour of specific growth rates in response to average daily light irradiance and temperature allowed the determination of optimal light and temperature conditions for the selected microalgae and cyanobacteria. This mathematical approach can be correctly applied to the selected microorganisms under light and temperature conditions within the range of those used in this study, providing the rapid determination of optimal growth conditions and reducing the time and costs associated to the optimization of culture parameters.
Acknowledgements
This work was financially supported by: Project UID/EQU/00511/2013-LEPABE, by the FCT/MEC with national funds and when applicable co-funded by FEDER in the scope of the P2020 Partnership Agreement; Project NORTE-07-0124-FEDER-000025 – RL2_Environment & Health, by FEDER funds through Programa Operacional Factores de Competitividade – COMPETE, by the Programa Operacional do Norte (ON2) program and by national funds through FCT – Fundação para a Ciência e a Tecnologia; SFRH/BD/88799/2012 and SFRH/BPD/66721/2009. The authors also acknowledge CIIMAR (Centre of Marine and Environmental Research of the University of Porto), for providing the cyanobacteria Synechocystis salina LEGE 06079 and Microcystis aeruginosa LEGE 91344.
References
- D. Tang, W. Han, P. Li, X. Miao and J. Zhong, Bioresour. Technol., 2011, 102, 3071–3076 CrossRef CAS PubMed.
- I. Rawat, R. Ranjith Kumar, T. Mutanda and F. Bux, Appl. Energy, 2011, 88, 3411–3424 CrossRef CAS.
- A. M. Silva-Benavides and G. Torzillo, J. Appl. Phycol., 2012, 24, 267–276 CrossRef CAS.
- L. Brennan and P. Owende, Renewable Sustainable Energy Rev., 2010, 14, 557–577 CrossRef CAS.
- Q. Hu, in Handbook of microalgal culture: biotechnology and applied phycology, ed. A. Richmond, Blackwell Science Ltd, Oxford, UK, 1st edn, 2004, ch. 12, pp. 268–271 Search PubMed.
- A. Parmar, N. K. Singh, A. Pandey, E. Gnansounou and D. Madamwar, Bioresour. Technol., 2011, 102, 10163–10172 CrossRef CAS PubMed.
- S. Singh, B. Kate and U. Banerjee, Crit. Rev. Biotechnol., 2005, 25, 73–95 CrossRef CAS PubMed.
- L. Barsanti and P. Gualtieri, Algae – Anatomy, Biochemistry and Biotechnology, CRC Press, USA, 2nd edn, 2006 Search PubMed.
- Q. Hu, in Handbook of microalgal culture: biotechnology and applied phycology, ed. A. Richmond, Blackwell Science Ltd, Oxford, UK, 1st edn, 2004, ch. 5, pp. 83–94 Search PubMed.
- A. Kumar, S. Ergas, X. Yuan, A. Sahu, Q. Zhang, J. Dewulf, F. X. Malcata and H. Van Langenhove, Trends Biotechnol., 2010, 28, 371–380 CrossRef CAS PubMed.
- H.-W. Y. Yen, I.-C. Hu, C.-Y. Chen and J.-S. Chang, in Biofuels from Algae, ed. A. Pandey, D.-J. Lee, Y. Chisti and C. R. Soccol, Elsevier, USA, 1st edn, 2013, ch. 2, pp. 23–46 Search PubMed.
- J. G. Sánchez, J. S. Pérez, F. G. Camacho, J. F. Sevilla and E. M. Grima, Biotechnol. Tech., 1996, 10, 329–334 Search PubMed.
- S. Chinnasamy, B. Ramakrishnan, A. Bhatnagar and K. C. Das, Int. J. Mol. Sci., 2009, 10, 518–532 CrossRef CAS PubMed.
- C. J. McLarnon-Riches, C. E. Rolph, D. L. Greenway and P. K. Robinson, Phytochemistry, 1998, 49, 1241–1247 CrossRef CAS.
- R. Philippis and M. Vincenzini, FEMS Microbiol. Rev., 1998, 22, 151–175 CrossRef.
- B. D. Wahlen, R. M. Willis and L. C. Seefeldt, Bioresour. Technol., 2011, 102, 2724–2730 CrossRef CAS PubMed.
- R. W. Eppley, Fish. Bull., 1972, 70, 1063–1085 Search PubMed.
- R. A. Parker, J. Fish. Res. Board Can., 1974, 31, 1550–1552 CrossRef CAS.
- J. Peeters and P. Eilers, Hydrobiol. Bull., 1978, 12, 134–136 CrossRef.
- J. H. Steele, in Chemical reactor theory, ed. L. Lapidus and N. R. Amunson, Prentice-Hall, Englewood Cliffs, New Jersey, 1977, vol. 7, pp. 405–483 Search PubMed.
- O. Bernard and B. Rémond, Bioresour. Technol., 2012, 123, 520–527 CrossRef CAS PubMed.
- A. P. Carvalho and F. X. Malcata, Biotechnol. Prog., 2003, 19, 1128–1135 CrossRef CAS PubMed.
- H. Haario, L. Kalachev and M. Laine, Bull. Math. Biol., 2009, 71, 1626–1648 CrossRef PubMed.
- OECD (Organisation for Economic Co-operation and Development), Freshwater alga and cyanobacteria, growth inhibition test, 2011.
- L. Wang, M. Min, Y. Li, P. Chen, Y. Chen, Y. Liu, Y. Wang and R. Ruan, Appl. Biochem. Biotechnol., 2010, 162, 1174–1186 CrossRef CAS PubMed.
- A. K. Pegallapati and N. Nirmalakhandan, Renewable Energy, 2013, 56, 129–135 CrossRef CAS.
- A. Gonçalves, M. Simões and J. Pires, Energy Convers. Manage., 2014, 85, 530–536 CrossRef.
- P. Feng, Z. Deng, L. Fan and Z. Hu, J. Biosci. Bioeng., 2012, 114, 405–410 CrossRef CAS PubMed.
- E. Jacob-Lopes, C. H. G. Scoparo, L. M. C. F. Lacerda and T. T. Franco, Chem. Eng. Process., 2009, 48, 306–310 CrossRef CAS.
- Y. Collos, F. Mornet, A. Sciandra, N. Waser, A. Larson and P. J. Harrison, J. Appl. Phycol., 1999, 11, 179–184 CrossRef.
- B. Lee, S. Y. Park, Y. S. Heo, S. S. Yea and D.-E. Kim, Bull. Korean Chem. Soc., 2009, 30, 2485–2488 CrossRef CAS.
- M. Wang, W. C. Kuo-Dahab, S. Dolan and C. Park, Bioresour. Technol., 2014, 154, 131–137 CrossRef CAS PubMed.
- J. M. Lee, Biochemical engineering, Prentice Hall, Englewood Cliffs, New Jersey, 1st edn, 1992 Search PubMed.
- A. Dauta, J. Devaux, F. Piquemal and L. Boumnich, Hydrobiologia, 1990, 207, 221–226 CrossRef.
- D. Schlesinger, L. Molot and B. Shuter, Can. J. Fish. Aquat. Sci., 1981, 38, 1052–1058 CrossRef.
- C. Sorokin and R. W. Krauss, Plant Physiol., 1958, 33, 109–113 CrossRef CAS PubMed.
- J. Uusitalo, Sci. Mar., 1996, 60, 129–134 CAS.
- C. James, S. Al-Hinty and A. Salman, Aquaculture, 1989, 77, 337–351 CrossRef CAS.
- S. H. Cho, S.-C. Ji, S. B. Hur, J. Bae, I.-S. Park and Y.-C. Song, Fish. Sci., 2007, 73, 1050–1056 CrossRef CAS.
- G.-J. Yang, Z.-Q. Luan, X.-H. Zhou and Y. Mei, J. Jap. Soc. Math. Phys. Fish. Sci., 2010, 8, 68–74 Search PubMed.
- C. Ugwu, H. Aoyagi and H. Uchiyama, Photosynthetica, 2007, 45, 309–311 CrossRef.
- F. Han, W. Wang, Y. Li, G. Shen, M. Wan and J. Wang, Bioresour. Technol., 2013, 132, 182–189 CrossRef CAS PubMed.
- Q. Hu, P. Westerhoff and W. Vermaas, Appl. Environ. Microbiol., 2000, 66, 133–139 CrossRef CAS PubMed.
- P. Talbot and J. De la Noüe, Water Res., 1993, 27, 153–159 CrossRef CAS.
- K. Lee and C.-G. Lee, Biotechnol. Bioprocess Eng., 2001, 6, 194–199 CrossRef CAS.
- Y. Li, W. Zhou, B. Hu, M. Min, P. Chen and R. R. Ruan, Biotechnol. Bioeng., 2012, 109, 2222–2229 CrossRef CAS PubMed.
- Y. Chisti, Biotechnol. Adv., 2007, 25, 294–306 CrossRef CAS PubMed.
- J. C. Goldman, Limnol. Oceanogr., 1977, 22, 932–936 CrossRef.
- S. H. Mortensen, K. Y. Børsheim, J. Rainuzzo and G. Knutsen, J. Exp. Mar. Biol. Ecol., 1988, 122, 173–185 CrossRef CAS.
- F.-X. Fu, M. E. Warner, Y. Zhang, Y. Feng and D. A. Hutchins, J. Phycol., 2007, 43, 485–496 CrossRef.
- R. Bouterfas, M. Belkoura and A. Dauta, Hydrobiologia, 2002, 489, 207–217 CrossRef.
- P. Claquin, I. Probert, S. Lefebvre and B. Veron, Aquat. Microb. Ecol., 2008, 51, 1–11 CrossRef.
- L. Aleya, A. Dauta and C. S. Reynolds, Eur. J. Protist., 2011, 47, 239–244 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26117a |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14, 14
14, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and 24
10, and 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (light
0 (light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark ratio). The light
dark ratio). The light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark ratio of 24
dark ratio of 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 was used because it promotes continuous photoautotrophic growth. To reduce production costs in terms of light requirements, the light
0 was used because it promotes continuous photoautotrophic growth. To reduce production costs in terms of light requirements, the light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark ratios of 10
dark ratios of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 and 14
14 and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 were applied to simulate the number of light hours during winter and summer time, respectively. For each studied condition, two independent experiments were performed. Taking into account the light irradiances and light
10 were applied to simulate the number of light hours during winter and summer time, respectively. For each studied condition, two independent experiments were performed. Taking into account the light irradiances and light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark ratios evaluated in this study, the corresponding average daily light irradiances are presented in Table 1.
dark ratios evaluated in this study, the corresponding average daily light irradiances are presented in Table 1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark ratio values applied to the selected cultures
dark ratio values applied to the selected cultures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark ratio (h
dark ratio (h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h)
h)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0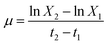
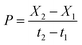
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500g for 10 min and supernatants were stored at −20 °C until being analysed. Nitrate concentration was determined through UV spectroscopy at 220 nm using a T80 UV/VIS Spectrophotometer (PG Instruments, UK), according to the method proposed by Collos et al.30 On the other hand, inorganic phosphate quantification was performed by measuring absorbance at 820 nm of a phosphomolybdate complex formed by reaction of inorganic phosphate with ammonium molybdate in a Synergy™ HT 96-well microplate reader (Biotek Instruments, Inc., USA), as proposed by Lee et al.31 Nutrients concentration in the first and last day of culturing were used to determine average removal rates (RR, in mgS L−1 d−1) and nutrients removal efficiencies (R, in %). Average removal rates were calculated as follows:32
500g for 10 min and supernatants were stored at −20 °C until being analysed. Nitrate concentration was determined through UV spectroscopy at 220 nm using a T80 UV/VIS Spectrophotometer (PG Instruments, UK), according to the method proposed by Collos et al.30 On the other hand, inorganic phosphate quantification was performed by measuring absorbance at 820 nm of a phosphomolybdate complex formed by reaction of inorganic phosphate with ammonium molybdate in a Synergy™ HT 96-well microplate reader (Biotek Instruments, Inc., USA), as proposed by Lee et al.31 Nutrients concentration in the first and last day of culturing were used to determine average removal rates (RR, in mgS L−1 d−1) and nutrients removal efficiencies (R, in %). Average removal rates were calculated as follows:32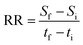





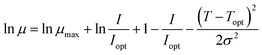

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)


