DOI:
10.1039/C5RA26112K
(Paper)
RSC Adv., 2016,
6, 20001-20013
A controlled, template-free, and hydrothermal synthesis route to sphere-like α-Fe2O3 nanostructures for textile dye removal
Received
7th December 2015
, Accepted 10th February 2016
First published on 12th February 2016
Abstract
Iron carbonate nanospheres were synthesized via hydrothermal treatment of aqueous solutions of iron sulfate, ascorbic acid and ammonium carbonate with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, respectively, at 140 °C for 1.5 h. Pure α-Fe2O3 nanoparticles with an average crystallite size of 10.5–32 nm were produced by thermal decomposition of FeCO3 at 400–600 °C for 2 h. The compositions of the products were identified by means of XRD, FE-SEM, HR-TEM, FT-IR, BET, zeta potential and thermal analysis. The adsorption properties of α-Fe2O3 were evaluated using reactive red 195 (RR195) dye. Various parameters influencing the adsorption process were investigated, using a batch technique. The results show that α-Fe2O3 nanoparticles show good adsorption capacity and the dye removal percentage reaches about 98.77% in 10 min. Plus, increasing the surface area of the α-Fe2O3 nanoparticles from 107.7 to 165.6 m2 g−1 increases the adsorption capacity from 4.7 to 20.5 mg g−1. Moreover, the adsorption data fit the Langmuir isotherm model well and the thermodynamic parameters exhibited an endothermic and spontaneous nature for the adsorption of RR195 dye on the hematite adsorbent.
3, respectively, at 140 °C for 1.5 h. Pure α-Fe2O3 nanoparticles with an average crystallite size of 10.5–32 nm were produced by thermal decomposition of FeCO3 at 400–600 °C for 2 h. The compositions of the products were identified by means of XRD, FE-SEM, HR-TEM, FT-IR, BET, zeta potential and thermal analysis. The adsorption properties of α-Fe2O3 were evaluated using reactive red 195 (RR195) dye. Various parameters influencing the adsorption process were investigated, using a batch technique. The results show that α-Fe2O3 nanoparticles show good adsorption capacity and the dye removal percentage reaches about 98.77% in 10 min. Plus, increasing the surface area of the α-Fe2O3 nanoparticles from 107.7 to 165.6 m2 g−1 increases the adsorption capacity from 4.7 to 20.5 mg g−1. Moreover, the adsorption data fit the Langmuir isotherm model well and the thermodynamic parameters exhibited an endothermic and spontaneous nature for the adsorption of RR195 dye on the hematite adsorbent.
1. Introduction
Over the past few years, water pollution has been considered as one of the major threats to our world because it results in an imbalance and significant alterations in the hydrological cycle which in turn cause many health problems and may even lead to death.1 Synthetic organic dyes mainly produced from different industries (such as leather, paper, textile, plastic, refineries, and chemical) are mostly toxic, carcinogenic, and stable against photodegradation and these dyes along with heavy metals induce water pollution.2,3 Hence, the wastewater generated from various industries has to be treated using an appropriate method before discharge, in order to get rid of these problems. There are several physical and chemical methods have been proposed for organic dye removal from wastewaters such as photodegradation, precipitation, filtration, coagulation, ion exchange, reverse osmosis, adsorption, etc.4–8 Among all the proposed attempts for dye removal, adsorption is still considered the most appropriate method in this concern due to its simplicity, economic applicability, high efficiency, and nonexistence of harmful species. Due to the high surface area of the nanomaterials, several research groups have focused their efforts on synthesizing of nanosized oxides using different routes and proposing them as good adsorbents.2–14
On the other hand, nanosized iron oxides have recently received a considerable interest of several research groups as efficient adsorbents for the decontamination purpose of wastewater.8,15,16 Among nano-sized iron(III) oxides, hematite, α-Fe2O3, is the most important and thermodynamically stable form under ambient conditions.17 Due to its stability, non-toxicity, high efficiency, and inexpensive nature, hematite has been used in different applications such as gas sensors, pigments, electrode materials, catalysts, magnetic resonance imaging, clinical therapy, magnetic materials, printing inks, ferrofluids, and water treatment.18–23 These interesting properties and applications stimulated many material and inorganic researchers to direct their efforts to synthesize α-Fe2O3 with different morphologies and particle sizes.24–27 As a consequence, several methods such as micro-emulsion, sol–gel, hydro/solvothermal, microwave-assisted solvothermal, ultrasonic precipitation, mechanochemical, thermal decomposition, and sono-electrochemical anodization have been developed for the synthesis of nanosized α-Fe2O3 structures.27–34 Although the methods that have been reported for synthesis of hematite are a lot, it is still a big challenge to have a facile and inexpensive methodology to control the morphology and particle size of the hematite nanostructures. So far, the hydrothermal technique still has the priority over the most synthetic methods due to its low-cost, simplicity, and varying morphologies of products.11 Plus, there are different parameters; such as temperature, time, solvents, and precursors, for the hydrothermal process the researcher can tune them to control the purity, particle size, and morphology of the products. However, we have reported on the hydrothermal synthesis of CoCO3 microspheres and their thermal conversion into cobalt oxide nanoparticles.5,11,12 One of the most important aspects of producing metal oxide nanoparticles through thermal conversion of metal carbonates is the formation of nano-pores due to the release of carbon dioxide from metal carbonate during the thermal decomposition step which results in novel nanostructures with relatively large specific surface area. Reports on similar synthetic routes for producing α-Fe2O3 via thermal decomposition of hydrothermally prepared ferrous carbonate are still limited.34–37 In such, Liu et al. reported on synthesis of FeCO3 microspheres using a surfactant-assisted hydrothermal reaction of iron chloride and urea.34 Plus, Xuan et al. prepared FeCO3 with still large particle size through the hydrothermal reaction of the same iron salt with different carbonate source (NaCO3).35 Also, Yang et al. reported on preparation of FeCO3 microspheres using the relatively inexpensive iron sulfate salt via its hydrothermal treatment with urea but they used relatively large quantity of ascorbic acid in their procedure and their FeCO3 have large particle size.36 However, an extensive study on the hydrothermal treatment of FeSO4 and (NH4)CO3 to produce FeCO3 with smaller particle size using smaller quantity of ascorbic acid at relatively low temperature has not been reported so far.
In the current work, as a continuation to our previous study,5,11,12 a facile hydrothermal synthesis was adopted, for the first time, to synthesize spherical FeCO3 nanoparticles in high yield by the reaction of iron sulfate and ammonium carbonate, as an inexpensive carbonate source, in presence of ascorbic acid. Different conditions affecting the synthesis process such as reaction temperature, molar ratio of the reactants, and reaction time have been investigated. Pure α-Fe2O3 nanoparticles are easily produced by thermal conversion of the as-prepared FeCO3 in air. The as-prepared materials have been characterized by means of thermal analysis and different spectroscopic tools. The applicability of the as-prepared hematite nanoparticles was investigated for the removal of Reactive Red 195 dye (RR195) as a pollutant model from aqueous phase.
2. Experimental
2.1. Chemicals and reagents
Unless otherwise stated, all chemicals and reagents in this work were of analytical grade and they were purchased and utilized as received without further purification: iron sulfate (FeSO4·7H2O; Sigma-Aldrich), ascorbic acid (C6H8O6; Sigma-Aldrich), and ammonium carbonate ((NH4)2CO3; Fluka), Reactive Red 195 dye (RR195) (C31H19ClN7O19S6Na5; Rushabh chemicals industries, India).
2.2. Preparation of FeCO3 and α-Fe2O3 nanoparticles
In a typical preparation procedure: iron sulfate (1.95 g, 7 mmol, 1 eq.) and ascorbic acid (1.23 g, 7 mmol, 1 eq.) were dissolved in 45 mL distilled water under stirring. To the stirring solution, an aqueous ammonium carbonate solution (2.016 g, 21 mmol, 3 eq.) (25 mL) was added and the stirring was continued for 10 min. Afterwards, the produced reaction mixture was then transferred into a 100 mL Teflon-lined autoclave which in turn was placed in an electric oven maintained at 140 °C for 1.5 h. The autoclave was naturally cooled and the precipitate (FeCO3) was centrifuged and washed several times with distilled water and ethanol and dried in an oven at ca. 60 °C overnight. However, in order to obtain the previously mentioned optimum conditions, different experimental parameters affecting this hydrothermal reaction such as temperature (120–160 °C), time (0.5–3 h), and molar ratio (iron sulfate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid
ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ammonium carbonate; 1
ammonium carbonate; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) -
-![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 1
3, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) have been investigated.
6) have been investigated.
The nanosized iron(II) carbonate product prepared at the optimized conditions of the hydrothermal process was calcined at 400, 500, or 600 °C for 2 h in air to produce α-Fe2O3 nanoparticles – with different crystallite sizes – denoted as Fe2O3-400, Fe2O3-500, and Fe2O3-600, respectively.
2.3. Characterization
The crystallinity and phase purity of the as-prepared products were identified via powder X-ray diffraction (XRD) by using 18 kW diffractometer (Bruker; model D8 Advance) with monochromated Cu-Kα radiation (l) 1.54178 Å. Using a field emission scanning electron microscope (FE-SEM) connected with a microscope (JEOL JSM-6500F), FE-SEM images were taken. The high resolution transmission electron microscope (HR-TEM) images were obtained on a transmission electron microscope (JEM-2100) at an accelerating voltage of 200 kV by dispersing the samples in ethanol ultrasonically on a copper grid. FT-IR spectra were recorded on FT-IR spectrometer (Thermo Scientific; model Nicolet iS10) from 4000 to 400 cm−1. The UV-vis spectra of adsorption studies were recorded using a Jasco UV-vis spectrophotometer (Jasco; model v670). The thermal analysis measurements of the as-prepared iron carbonate were carried out on a thermal analyzer instrument (Shimadzu; model TA-60WS) in a nitrogen atmosphere with a heating rate of 15 °C min−1. BET (Brunauer–Emmet–Teller) surface area of the as-synthesized Fe2O3 products was performed on Quantachrome (Nova 2000 series, USA) using nitrogen adsorption–desorption measurements. The zeta potential of the nanosized hematite product was measured using a Zetasizer nano series meter (Nano ZS, Malvern, UK).
2.4. Adsorption experiments
The adsorption experiments were carried out in a batch method performed under a continuous magnetic stirring using Reactive Red 195 dye (RR195) as an adsorbate model, Table 1. The RR195 dye is a commercial product and it is used as received from Rushabh chemicals industries company, India, without further purification. Different experimental factors affecting the adsorption process such as dye solution pH (0.5–9), contact time (5–70 min), temperature (25–45 °C), initial concentration (10–50 ppm) and salt effect, have been investigated. In a typical batch experiment, 50 mL of an aqueous RR195 dye solution of a desired concentration was prepared by suitable dilution of a stock solution and by using 0.1 mol L−1 HCl or NaOH aqueous solutions the pH of the solution was adjusted. Afterwards, a known quantity (0.1 g) of dry α-Fe2O3 nanoparticles was added and the produced suspension in the conical flask was then allowed to magnetically stir (400 rpm) for a definite time at room temperature. According to the experimental conditions under investigation, the temperatures and concentrations of the adsorbate were varied. After a predetermined time of stirring, an aliquot was taken out of the flask and the adsorbent was separated by centrifugation at 4000 rpm for about 4 min and then the concentration of the remaining dye in the supernatant solution was determined using UV-vis spectrophotometer. It is noteworthy that analysis of the remaining dye concentration in the supernatant was carried out using a previously constructed calibration graph. The obtained data were used to calculate the adsorption capacities (qt, mg g−1) and the dye removal efficiencies (% removal) using eqn (1) and (2).| |
 | (1) |
| |
 | (2) |
where C0 and Ct are the RR195 dye concentrations in mg L−1 at time = 0 and t respectively in solution, V is the volume of dye solution (L), and m is the mass of dry α-Fe2O3 adsorbent (g).
Table 1 Characteristics and chemical structure of reactive red 195 dye
| Dye name |
Reactive red 195, RR195 |
| Chemical structure |
 |
| Molecular formula |
C31H19ClN7O19S6Na5 |
| Chemical class |
Azo derivative compound |
| λmax |
542 nm |
| Type |
Anionic dye |
| Solubility |
Water soluble |
| C.I. number |
Reactive red 195 |
3. Results and discussion
3.1. Hydrothermal synthesis and characterization of FeCO3 nanospheres
Hydrothermal reactions of iron sulfate and ammonium carbonate in presence of ascorbic acid have been investigated in order to prepare pure iron carbonate. Fig. 1(a) exhibits the XRD pattern of FeCO3 nanospheres synthesized by the hydrothermal treatment of aqueous solutions of iron sulfate, ammonium carbonate and ascorbic acid at the optimal conditions: Fe2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid
ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO32− molar ratio of 1
CO32− molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1.5 h reaction time, and 140 °C reaction temperature. All the diffraction peaks can be indexed well to a pure rhombohedral phase of FeCO3 which is in consistent with the standard patterns of iron carbonate (space group R
3, 1.5 h reaction time, and 140 °C reaction temperature. All the diffraction peaks can be indexed well to a pure rhombohedral phase of FeCO3 which is in consistent with the standard patterns of iron carbonate (space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c, JCPDS card 83-1764).37 No other characteristic peaks corresponding to iron oxides or even other impurities have been observed. The average crystallite size (D) of the as-prepared FeCO3 was found to be 22 nm which was calculated using the Debye–Scherrer formula:38
c, JCPDS card 83-1764).37 No other characteristic peaks corresponding to iron oxides or even other impurities have been observed. The average crystallite size (D) of the as-prepared FeCO3 was found to be 22 nm which was calculated using the Debye–Scherrer formula:38
D = 0.9λ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θB θB |
where λ is the X-ray wavelength, β is the full width at half maximum (FWHM) of the diffraction peak and θB is the Bragg diffraction angle. Producing of α-Fe2O3 nanoparticles, Fig. 1(b), was performed through thermal conversion of the hydrothermally as-synthesized iron carbonate precursor at the optimal condition as will be explained later.
 |
| | Fig. 1 XRD patterns of the as-synthesized FeCO3 (a), and α-Fe2O3 nanoparticles produced at: 400 °C (b), 500 °C (c), and 600 °C (d). | |
3.1.1. Optimization of the hydrothermal preparation of FeCO3 nanoparticles. The optimum conditions for preparation of pure iron carbonate have been reached by studying the various reaction conditions influencing the hydrothermal process such as reaction temperature, reaction time and iron sulfate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid
ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ammonium carbonate molar ratio using the XRD analysis.
3.1.1.1. Effect of temperature. Effect of temperature on the hydrothermal treatment of the reactants has been investigated first and Fig. 2(a)–(c) exhibits the XRD patterns of the hydrothermally produced products using molar ratio of 1
ammonium carbonate molar ratio using the XRD analysis.
3.1.1.1. Effect of temperature. Effect of temperature on the hydrothermal treatment of the reactants has been investigated first and Fig. 2(a)–(c) exhibits the XRD patterns of the hydrothermally produced products using molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 for iron sulfate
6 for iron sulfate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid
ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ammonium carbonate, respectively, for 3 h at different temperatures (160, 140, and 120 °C). It can be noticed that the three temperatures produced iron carbonates and the optimum temperature was 140 °C since it gave pure iron carbonate with a moderate crystallite size (80 nm). However, 160 °C temperature gave pure product with very large crystallite size (200 nm), and 120 °C produced the product with very poor crystallinity contaminated with some impurities as indicated from their XRD patterns and appearance of some peaks for some undefined by-products.
ammonium carbonate, respectively, for 3 h at different temperatures (160, 140, and 120 °C). It can be noticed that the three temperatures produced iron carbonates and the optimum temperature was 140 °C since it gave pure iron carbonate with a moderate crystallite size (80 nm). However, 160 °C temperature gave pure product with very large crystallite size (200 nm), and 120 °C produced the product with very poor crystallinity contaminated with some impurities as indicated from their XRD patterns and appearance of some peaks for some undefined by-products.
 |
| | Fig. 2 (a–c) XRD patterns of the FeCO3 samples prepared under hydrothermal conditions for 3 h with molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 for iron sulfate 6 for iron sulfate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ammonium carbonate, respectively, at different temperatures: 120 (a), 140 (b), and 160 °C (c). ammonium carbonate, respectively, at different temperatures: 120 (a), 140 (b), and 160 °C (c). | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid
ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ammonium carbonate molar ratio of 1
ammonium carbonate molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. The results indicated that the optimum reaction time was 1.5 h. On the other hand, shorter time (i.e. <1.5 h) produced iron carbonate with poor crystallinity and low yield, but longer reaction time gave particles with very large crystallite size. On comparing the optimum temperature and time in the current study with other reported ones for preparing pure iron carbonate, it can easily be seen that we have used lower temperature and shorter time than the reported ones.34–36
6. The results indicated that the optimum reaction time was 1.5 h. On the other hand, shorter time (i.e. <1.5 h) produced iron carbonate with poor crystallinity and low yield, but longer reaction time gave particles with very large crystallite size. On comparing the optimum temperature and time in the current study with other reported ones for preparing pure iron carbonate, it can easily be seen that we have used lower temperature and shorter time than the reported ones.34–36
 |
| | Fig. 3 (a–e) XRD patterns of the samples prepared under hydrothermal conditions with molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 for iron sulfate 6 for iron sulfate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ammonium carbonate, respectively, at 140 °C for 0.5 (a), 1 (b), 1.5 (c), 2 (d), and 3 h (e). ammonium carbonate, respectively, at 140 °C for 0.5 (a), 1 (b), 1.5 (c), 2 (d), and 3 h (e). | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO32− molar ratio of 1
CO32− molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 in absence of ascorbic acid, and the XRD pattern of the product is shown in Fig. 4(a). Also, the influence of addition of different quantities of ascorbic acid have been explored by trying different Fe2+
6 in absence of ascorbic acid, and the XRD pattern of the product is shown in Fig. 4(a). Also, the influence of addition of different quantities of ascorbic acid have been explored by trying different Fe2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid
ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO32− molar ratios: 1
CO32− molar ratios: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, and 1
6, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6; and the XRD results are depicted in Fig. 4(b)–(e), respectively. Inspection of XRD patterns of the produced products (Fig. 4) reveals that the minimum required equivalents of ascorbic acid are 1 (i.e. Fe2+
6; and the XRD results are depicted in Fig. 4(b)–(e), respectively. Inspection of XRD patterns of the produced products (Fig. 4) reveals that the minimum required equivalents of ascorbic acid are 1 (i.e. Fe2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid
ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO32− optimum molar ratio is 1
CO32− optimum molar ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) and using less equivalents of the ascorbic acid (i.e. <1 equivalent) gives iron carbonate with poor crystallinity along with some impurities such as magnetite. It is worthy to mention that in the current hydrothermal treatment smaller quantity of ascorbic acid has been used to produce pure iron carbonate with smaller particle size on comparing to the other reported methods.34–36 However, the mechanism of the current hydrothermal process can be explained according to the proposed Scheme 1 as follows: ammonium carbonate and iron sulfate generate carbonate and iron ions according to reactions (i) and (ii), respectively. At the optimum or higher concentrations of both carbonate ions and ascorbic acid, reactions (iii) and (vii) (Scheme 1) will be the more predominant reactions and accordingly the product will be pure iron carbonate. At lower concentration of ascorbic acid, Fe3+ ions (produced from oxidation of Fe2+ during mixing or heating) will not be completely reduced to Fe2+ ions (reaction (iv)) and in turn Fe3+ ions will react with OH− (which can be generated from reaction (v)) to produce Fe(OH)3. Thence, there will be a competition between reactions (iii), (vii) and (ix) and the result of that competition will be a mixture of iron carbonate and Fe3O4. Additionally, Fe3O4 impurities are by-products generated from the reaction between Fe(OH)3 (reaction (viii)) and Fe(OH)2 (reaction (vi)) at the hydrothermal conditions according to reaction (ix) (Scheme 1) and this is in agreement with the reported data.36
6) and using less equivalents of the ascorbic acid (i.e. <1 equivalent) gives iron carbonate with poor crystallinity along with some impurities such as magnetite. It is worthy to mention that in the current hydrothermal treatment smaller quantity of ascorbic acid has been used to produce pure iron carbonate with smaller particle size on comparing to the other reported methods.34–36 However, the mechanism of the current hydrothermal process can be explained according to the proposed Scheme 1 as follows: ammonium carbonate and iron sulfate generate carbonate and iron ions according to reactions (i) and (ii), respectively. At the optimum or higher concentrations of both carbonate ions and ascorbic acid, reactions (iii) and (vii) (Scheme 1) will be the more predominant reactions and accordingly the product will be pure iron carbonate. At lower concentration of ascorbic acid, Fe3+ ions (produced from oxidation of Fe2+ during mixing or heating) will not be completely reduced to Fe2+ ions (reaction (iv)) and in turn Fe3+ ions will react with OH− (which can be generated from reaction (v)) to produce Fe(OH)3. Thence, there will be a competition between reactions (iii), (vii) and (ix) and the result of that competition will be a mixture of iron carbonate and Fe3O4. Additionally, Fe3O4 impurities are by-products generated from the reaction between Fe(OH)3 (reaction (viii)) and Fe(OH)2 (reaction (vi)) at the hydrothermal conditions according to reaction (ix) (Scheme 1) and this is in agreement with the reported data.36
 |
| | Fig. 4 (a–e) XRD patterns of the samples prepared under hydrothermal conditions with Fe2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO32− molar ratios of 1 CO32− molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (a), and with Fe2+ 6 (a), and with Fe2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO32− molar ratios of 1 CO32− molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (b), 1 6 (b), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (c), 1 6 (c), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (d), and 1 6 (d), and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (e), at 140 °C for 1.5 h. 6 (e), at 140 °C for 1.5 h. | |
 |
| | Scheme 1 Proposed reaction mechanism for formation of FeCO3 or a mixture of FeCO3 and Fe3O4. | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) CO32− molar ratios. Effect of ammonium carbonate concentration has been investigated by keeping Fe2+ ion and ascorbic acid concentrations constant and varying the concentration of ammonium carbonate by using the following different molar ratios: 1
CO32− molar ratios. Effect of ammonium carbonate concentration has been investigated by keeping Fe2+ ion and ascorbic acid concentrations constant and varying the concentration of ammonium carbonate by using the following different molar ratios: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 1
3, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 for Fe2+
6 for Fe2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid
ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO32−, respectively, and the XRD patterns of the products are given in Fig. 5(a)–(d), respectively. The obtained results reveal that using lower concentration of CO32− anion than 3 equivalents produces by-products along with FeCO3 with very poor crystallinity. Consequently, it can be concluded that the optimum molar ratio for this template-free hydrothermal reaction is (1
CO32−, respectively, and the XRD patterns of the products are given in Fig. 5(a)–(d), respectively. The obtained results reveal that using lower concentration of CO32− anion than 3 equivalents produces by-products along with FeCO3 with very poor crystallinity. Consequently, it can be concluded that the optimum molar ratio for this template-free hydrothermal reaction is (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) for Fe2+
3) for Fe2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid
ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO32−, respectively.
CO32−, respectively.
 |
| | Fig. 5 (a–d) XRD patterns of the samples prepared under hydrothermal conditions with Fe2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO32− molar ratios of 1 CO32− molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (a), 1 1 (a), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (b), 1 2 (b), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (c), and 1 3 (c), and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (d), at 140 °C for 1.5 h. 6 (d), at 140 °C for 1.5 h. | |
3.1.2. Morphological study. Fig. 6(Ia and Ib) presents the FE-SEM images of the as-synthesized FeCO3 nanostructures prepared under the optimized conditions with a low and high magnifications, respectively. It is obvious from the low magnification image, Fig. 6(I), that the iron carbonate product is almost composed of agglomerates of nanospheres and from the high magnification image, Fig. 6(Ib), average size of the nanospheres was estimated to be about 70 nm in the diameter. It is noticeably that pure iron carbonate with smaller particle size (70 nm) has been successfully synthesized hydrothermally on comparing to the previously reported results.34–36
 |
| | Fig. 6 FE-SEM images for as-synthesized FeCO3 (Ia, Ib), and α-Fe2O3 produced at 600 °C (IIa, IIb); low (Ia, IIa) and high (Ib, IIb) magnification images. | |
3.1.3. FT-IR study. Fig. 7(a) displays the IR spectrum of the as-synthesized FeCO3 product and it reveals vibrational absorptions at 1392, 1109, 859, and 735 cm−1 attributing to the absorbance of pure iron carbonate product and these stretching vibrations are fingerprint of D3h symmetry which in turn is an evidence for the presence of carbonate anions in the iron carbonate products.5,11,12,39 The weak band appeared at 2457 cm−1 may be assigned to the stretching vibration of the carbonate anions.5,11,12,40 Plus, the vibrational shoulder observed at 1780 cm−1 may be ascribed to the overtone or combination of some vibrational frequencies of divalent metal cations and the carbonate group bond.5,11,12,40 The broad vibrational band at 3140 cm−1 and the band at 1660 cm−1 may be assigned to the stretching and the bending vibrations, respectively, of adsorbed water molecules interacting with CO32− anions of the iron carbonate molecules.5,11,12,34
 |
| | Fig. 7 FT-IR spectra of the as-synthesized FeCO3 (a), and α-Fe2O3 produced at 600 °C (b). | |
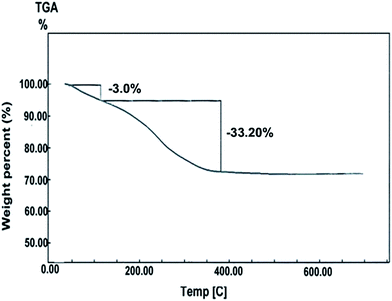
3.1.4. Thermal study. The thermal conversion of the as-synthesized iron carbonate product was investigated using TG analysis, Fig. 8. Fig. 8 indicates the presence of 36.20% overall weight loss in two weight loss steps in the TG curve in the temperature ranges 25–100 °C and 120–350 °C. The first weight loss, ca. 3.0% (theoretical value, 3.75%), may be assigned to the loss of adsorbed/trapped water molecules from the iron carbonate sample. Whereas, the second step, 33.20% (theoretical value, 33.66%) may be due to the thermal conversion of FeCO3 into Fe2O3, CO, and CO2 under N2 gas atmosphere. This thermal behavior is similar to the that reported for CoCO3 elsewhere.5,11,12
 |
| | Fig. 8 Thermo-gravimetric analysis of the as-synthesized FeCO3 under N2 gas. | |
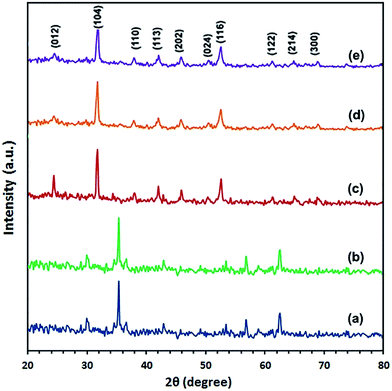
3.2. Preparation and characterization of α-Fe2O3 nanoparticles
Based on the aforementioned thermal data, the as-synthesized iron carbonate nanospheres, prepared at the optimized conditions, were thermally heated up at 400 °C for 2 h to produce pure α-Fe2O3 nanoparticles. Fig. 1(b) exhibits the XRD pattern of the iron oxide product and this pattern can be readily indexed to pure α-Fe2O3 phase with the hexagonal structure (space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c, lattice constant; a = 5.038 Å and c = 13.776 Å, JCPDS card 89-0598). The estimated average crystallite size of the produced α-Fe2O3 nanoparticles using the Debye–Scherrer equation38 was found to be ca. 10.5 nm. Moreover, the as-prepared FeCO3 precursor was also calcinated at 500 and 600 °C to α-Fe2O3 nanoparticles with different crystallite sizes; 19.8 and 31.2 nm, respectively, calculated from the XRD patterns of the produced α-Fe2O3, Fig. 1(c) and (d), using the Debye–Scherrer equation. It is noticeable that higher calcination temperatures produced larger crystallite sizes. The surface morphology and microstructure of α-Fe2O3 particles produced at 600 °C and displayed in Fig. 6(II) and 9 have been investigated using FE-SEM and TEM, respectively. It can be seen from Fig. 6(II) that α-Fe2O3 product is clusters of individually spherical shape α-Fe2O3 particles and this indicates that iron carbonate morphologies on calcination have almost been retained for α-Fe2O3 product. Inspection the size and morphology of the α-Fe2O3 particles, Fig. 9, using TEM images exhibits that the α-Fe2O3 product consists of irregular and spherical morphology with an average particle size of ca. 32 nm which is consistent with the one obtained using XRD data. Chemical structure of α-Fe2O3 produced at 600 °C (Fe2O3-600 product) was also confirmed by using Fourier transform infrared spectrum, Fig. 7(b), which exhibited two strong absorption bands at 547.7 and 461.9 cm−1 corresponding to the Fe–O bond which is consistent with the published data.21,41,42 Plus, the two bands appeared at 3370 and 1600 cm−1 can be attributed to the stretching and bending vibrations of adsorbed surface water molecules interacting with the oxide product and the broadness of these peaks may be assigned to hydrogen bonding O–H.39
c, lattice constant; a = 5.038 Å and c = 13.776 Å, JCPDS card 89-0598). The estimated average crystallite size of the produced α-Fe2O3 nanoparticles using the Debye–Scherrer equation38 was found to be ca. 10.5 nm. Moreover, the as-prepared FeCO3 precursor was also calcinated at 500 and 600 °C to α-Fe2O3 nanoparticles with different crystallite sizes; 19.8 and 31.2 nm, respectively, calculated from the XRD patterns of the produced α-Fe2O3, Fig. 1(c) and (d), using the Debye–Scherrer equation. It is noticeable that higher calcination temperatures produced larger crystallite sizes. The surface morphology and microstructure of α-Fe2O3 particles produced at 600 °C and displayed in Fig. 6(II) and 9 have been investigated using FE-SEM and TEM, respectively. It can be seen from Fig. 6(II) that α-Fe2O3 product is clusters of individually spherical shape α-Fe2O3 particles and this indicates that iron carbonate morphologies on calcination have almost been retained for α-Fe2O3 product. Inspection the size and morphology of the α-Fe2O3 particles, Fig. 9, using TEM images exhibits that the α-Fe2O3 product consists of irregular and spherical morphology with an average particle size of ca. 32 nm which is consistent with the one obtained using XRD data. Chemical structure of α-Fe2O3 produced at 600 °C (Fe2O3-600 product) was also confirmed by using Fourier transform infrared spectrum, Fig. 7(b), which exhibited two strong absorption bands at 547.7 and 461.9 cm−1 corresponding to the Fe–O bond which is consistent with the published data.21,41,42 Plus, the two bands appeared at 3370 and 1600 cm−1 can be attributed to the stretching and bending vibrations of adsorbed surface water molecules interacting with the oxide product and the broadness of these peaks may be assigned to hydrogen bonding O–H.39
 |
| | Fig. 9 HR-TEM image of the as-produced α-Fe2O3 nanoparticles produced at 600 °C. | |
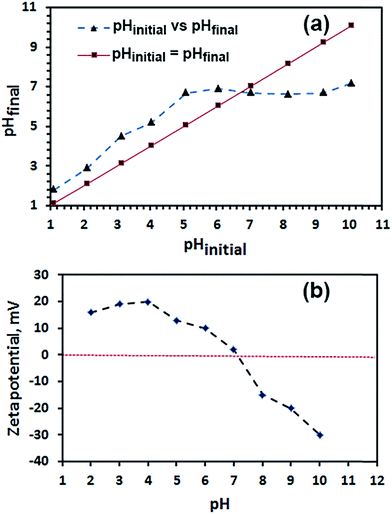
3.2.1. Point of zero charge (PZC) and isoelectric point (IEP). The point of zero charge (pHpzc) of Fe2O3 nanoparticles calcinated at 600 °C (Fe2O3-600) was estimated by the pH drift method as reported by Ahmaruzzaman et al.43 It is notably, pHpzc is the pH at which charge on the Fe2O3 nanoparticles is zero. The pHpzc of the Fe2O3 was determined as follows: 25 mLs of 0.01 M NaCl solution with initial pH (pHinitial) in the range between 2.0 and 10.0 adjusted with 0.1 M NaOH or 0.1 M HCl solution. Then, each NaCl solution (25 mL) was added to 0.05 g of the Fe2O3 adsorbent then left to stir for 48 h. The mixture was centrifuged and pH of the supernatant was measured (pHfinal). Fig. 10(a), exhibits plotting of pHinitial versus pHfinal and the point at which the curve pHinitial − pHfinal intersects the pHinitial = pHfinal line is the pHpzc. The pHpzc value of the Fe2O3 adsorbent is estimated to be ca. 6.8 which is in agreement with the published data.44,45
 |
| | Fig. 10 pHinitial against pHfinal plot for determination of pHpzc for of α-Fe2O3 nanoparticles (a), and zeta potential of α-Fe2O3 nanoparticles as a function of pH (b). | |
On the other hand, the pH of the isoelectric point (IEP) was determined by plotting the zeta potentials of Fe2O3 suspensions in 0.01 M NaCl solutions at various pH range from 2 to 10, as shown in Fig. 10(b). The pH was adjusted with 0.1 M NaOH or 0.1 M HCl solutions. The pH value of the isoelectric point (IEP) is found to be ca. 7.1 which is in consistent with the published data.44–46
3.3. Adsorption properties of α-Fe2O3 nanoparticles
Adsorption properties of the α-Fe2O3 nanoparticles calcinated at 600 °C (Fe2O3-600) were investigated by choosing RR195 dye as an adsorbate model.
3.3.1. Effect of pH. Initial pH of the solution plays an important role in the adsorption experiments because it influences the surface binding sites of the nanoparticles acting as adsorbents and the dye speciation, as well. Effect of the initial pH of the solution on the adsorption of the RR195 dye (C0 = 20 ppm, λmax = 542 nm) on α-Fe2O3 nanoparticles (m = 0.1 g) for 24 h as contact time was investigated and presented in Fig. 11. The data exhibited that the adsorption was pH dependent and the maximum adsorption was observed to be in the range of pH 1–3. Afterwards, the adsorption decreased slowly by increasing pH up to almost 5 then decreased significantly at higher pH values. Therefore, the other adsorption experiments in this study were carried out at pH 2, as an optimized pH value. This phenomenon may be explained on the basis that at pHs lower than pHpzc or IEP (pHpzc = 6.8 and IEP = 7.1 for α-Fe2O3 nanoparticles), the surface of the α-Fe2O3 is positively charged and hence the attraction between the oppositely charges (i.e. these positively charges and the negatively charged RR195 dye) results in higher adsorption. Consequently, at pHs higher than pHpzc or IEP there will be an electrostatic repulsion between the negatively charged α-Fe2O3 surface and the negatively charged RR195 dye and this may be the reason for lower adsorption at higher pH values.
 |
| | Fig. 11 pH influence on RR195 dye removal efficiency. | |
3.3.2. Effect of initial dye concentration. The dependence of the adsorption on the RR195 dye initial concentration (10–50 ppm) was investigated at room temperature and pH 2 and presented in Fig. 12. It can be clearly observe that the removal efficiency decreases from 95.5% to 42.6% with increasing the initial dye concentration from 10 ppm to 50 ppm, respectively. This may be attributed to saturation of the adsorption surface sites on the α-Fe2O3 adsorbent on increasing the initial dye concentration.
 |
| | Fig. 12 Influence of initial RR195 dye concentration on its removal percentage. | |
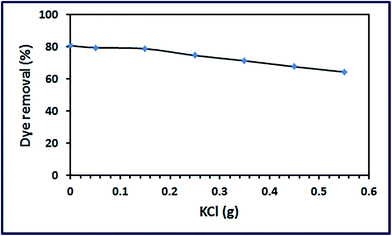
3.3.3. Effect of ionic strength. The presence of dissolved inorganic ions is well known problem in wastewater containing industrial dyes.47 These dissolved ions can compete with the dye of interest for the adsorption on the active sites on the adsorbent surface and hence this may reduce the adsorption efficiency of the dyes. In this study, influence of ionic strength by addition of KCl salt to the dye solution (30 ppm) on the adsorption process was investigated and presented in Fig. 13. The data indicate the decrease of the dye removal by increasing the KCl concentration because ions of the salt compete with the RR195 dye in adsorption by α-Fe2O3 adsorbent.
 |
| | Fig. 13 Effect of KCl concentration on RR195 dye removal percentage. | |
3.3.4. Effect of contact time on the adsorption and the kinetic studies. The effect of contact time on the adsorption of RR195 dye was investigated at the optimized pH 2, 0.1 g adsorbent, and 10 ppm dye initial concentration, as shown in Fig. 14. It can be seen that the adsorption amount increased rapidly and reached about 95.5% in only 10 min and afterwards it stayed almost constant. Hence, 10 min was selected as the optimized contact time for the remaining study. This rapid adsorption may be attributed to the presence of large number of surface sites for the α-Fe2O3 nanoparticles available for adsorption.
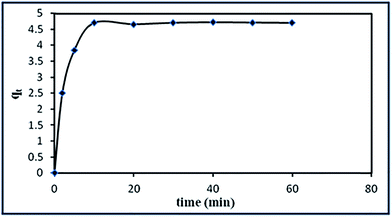 |
| | Fig. 14 Influence of contact time on RR195 dye adsorption by α-Fe2O3 nanoparticles. | |
To gain some information about the adsorbate amount and the rate of the adsorption process, kinetic parameters of RR195 dye adsorption on the α-Fe2O3 adsorbent were calculated by applying pseudo-first order equation,48 pseudo-second order equation,49 and intraparticle diffusion model,50 as shown in Fig. 15(a)–(c), respectively. It is noteworthy that the validity and fitting of the kinetic model can be examined by checking the value of the linear regression which is also known as a correlation coefficient, r2 . The linear form of pseudo-first order kinetic model is:
| |
 | (3) |
where
k1 (min
−1) is the pseudo-first order rate adsorption constant,
qe (mg g
−1) is the adsorption capacity at equilibrium, and
qt (mg g
−1) is the adsorption capacity at time
t (min). Plus, values of
k1 can be determined from plotting of log(
qe −
qt) against
t,
Fig. 15(a). However, the obtained experimental data did not fit the pseudo-first order kinetic model, and the straight line could not be obtained, hence we tried to fit the adsorption data using pseudo-second order kinetic model. The linear form of the pseudo-second order kinetic equation can be given as follows:
| |
 | (4) |
where
k2 ((g mg
−1) min) is the rate constant of pseudo-second order rate adsorption constant which can be calculated from plotting of
t/
qt against
t as shown in
Fig. 15(b). The adsorption kinetic constants including the calculated equilibrium adsorption capacity
qe(cal) and the experimental equilibrium adsorption capacity
qe(exp) for the adsorption of RR195 dye on α-Fe
2O
3 adsorbent are presented in
Table 1. Based on
Fig. 15(a) and (b) and the values of the regression coefficients presented in
Table 2, it is clearly obvious that the adsorption of RR195 dye on α-Fe
2O
3 adsorbent can be described well by pseudo-second order model. Plus, the experimental adsorption capacity value (
qe(exp)) is consistent with the calculated one (
qe(cal)). However, in order to gain further insight into the rate determining step involved in the adsorption of RR195 dye on α-Fe
2O
3 adsorbent, intra-particle diffusion model was examined by applying the Weber and Morris equation:
where
ki is the intra-particle diffusion constant (mg (g min
1/2)
−1), and
C represents the boundary layer thickness (mg g
−1). According to this model, plotting of
qt values against
t0.5 values should give a straight line which passes through the origin, if the rate determining step is the intra-particle diffusion. Otherwise, boundary layer diffusion or chemical reaction is the rate determining step. In the current case, the obtained plot (
Fig. 15(c)) is a multilinear and does not passing the origin which indicates that the overall adsorption process may proceed by more than one mechanism such as film diffusion, chemical reaction, and intra-particle diffusion.
51
 |
| | Fig. 15 Adsorption kinetic; (a) pseudo-first-order, (b) pseudo-second-order, and (c) intra-particle diffusion model, for RR195 dye adsorption onto α-Fe2O3 nanoparticles. | |
Table 2 Kinetic constants for RR195 dye adsorption on α-Fe2O3 nanoparticles
| Kinetics models |
Parameters |
Value |
| Pseudo-first order |
k1 (min−1) |
0.036 |
| qe(cal) (mg g−1) |
4.70 |
| r12 |
0.339 |
| qe(exp) (mg g−1) |
0.042 |
| Pseudo-second order |
k2 [g mg−1 min−1] |
0.208 |
| qe(cal) (mg g−1) |
4.70 |
| r22 |
0.999 |
| qe(exp) (mg g−1) |
4.81 |
3.3.5. Adsorption isotherms. Adsorption results have been analyzed using the two well-known isotherms; Langmuir and Freundlich models. These two models can be applied to describe the adsorption capacity of the adsorbent at different equilibrium concentrations of the dye at the optimized adsorption conditions. The linear form of the Langmuir relationship can be expressed as follows:| |
 | (6) |
where Ce is the equilibrium concentration of the RR195 dye in solution (mg L−1), qe is the equilibrium adsorption capacity of the RR195 dye on the α-Fe2O3 adsorbent, qmax is the maximum of adsorbed solute to form a monolayer per gram of adsorbent (mg g−1), and KL is the Langmuir adsorption constant (L mg−1). The constants KL and qmax can be calculated from the slope and intercept of the linear plot of Ce/qe versus Ce (Fig. 16(a)). The linear form of the Freundlich isotherm can be given as follows:| |
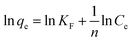 | (7) |
where n and KF are Freundlich constants related to adsorption intensity and adsorption capacity of the α-Fe2O3 adsorbent, respectively, and they are obtained from the slope and intercept of the linear plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe against ln
qe against ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce (Fig. 16(b)). The Langmuir and Freundlich constants are presented in Table 3. Clearly, the adsorption of RR195 dye on the α-Fe2O3 adsorbent follows the Langmuir adsorption isotherm model and the correlation coefficient for this model is estimated to be 0.992. Plus, fitting of the experimental data to Langmuir isotherm model exhibits the homogeneous nature of α-Fe2O3 surface which in turn means that each RR195 dye molecule/adsorption has equal activation energy of adsorption. Hence, the results reveal that the adsorption of the RR195 dye is monolayer coverage at the outer surface of α-Fe2O3 adsorbent. Moreover, the efficiency of the adsorption of the RR195 dye on the adsorbent can be estimated through the values of the separation factor constant (RL) which can be given as follows:
Ce (Fig. 16(b)). The Langmuir and Freundlich constants are presented in Table 3. Clearly, the adsorption of RR195 dye on the α-Fe2O3 adsorbent follows the Langmuir adsorption isotherm model and the correlation coefficient for this model is estimated to be 0.992. Plus, fitting of the experimental data to Langmuir isotherm model exhibits the homogeneous nature of α-Fe2O3 surface which in turn means that each RR195 dye molecule/adsorption has equal activation energy of adsorption. Hence, the results reveal that the adsorption of the RR195 dye is monolayer coverage at the outer surface of α-Fe2O3 adsorbent. Moreover, the efficiency of the adsorption of the RR195 dye on the adsorbent can be estimated through the values of the separation factor constant (RL) which can be given as follows:| |
 | (8) |
where C0, and KL are the initial concentration (mg L−1) and the Langmuir constant (L mg−1), respectively. The value of the RL indicates whether the isotherm type is irreversible RL = 0, favorable 0 < RL < 1, linear RL = 1, or unfavorable RL > 1. The calculated RL value in the current case is found to be 0.00615 which refers to that the adsorption of the RR195 dye on the α-Fe2O3 adsorbent is favorable in this investigation at the optimized adsorption conditions.
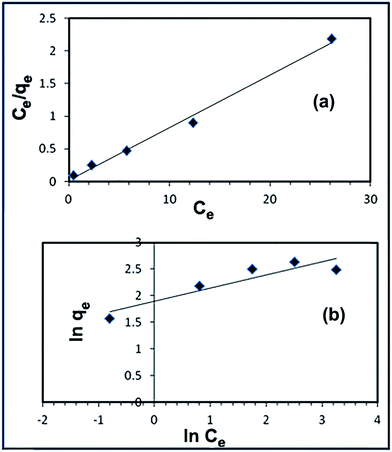 |
| | Fig. 16 Langmuir (a) and Freundlich (b) isotherms for RR195 dye adsorption onto α-Fe2O3 nanoparticles. | |
Table 3 Isotherm parameters for RR195 dye adsorption of on α-Fe2O3 nanoparticles
| Isotherm models |
Constants |
Value |
| Langmuir |
KL (L mg−1) |
3.23 |
| qm(cal) (mg g−1) |
4.87 |
| r12 |
0.992 |
| RL |
0.0062 |
| qe(exp) (mg g−1) |
4.70 |
| Freundlich |
KF (mg g−1) (L mg−1)1/n |
6.65 |
| qe(cal) (mg g−1) |
20.7 |
| r22 |
0.844 |
| n |
4.02 |
| qe(exp) (mg g−1) |
4.70 |
3.3.7. Surface area influence on the adsorption process and Fe2O3 adsorbent reusability. One of the factors that can influence on the adsorption process is the surface area of the adsorbents. The BET surface area (using nitrogen adsorption–desorption isotherms), BJH pore size, total pore volume, and crystallite size (calculated from the Debye–Scherrer equation) of the nano-sized products (Fe2O3-400, Fe2O3-500, and Fe2O3-600) were investigated and the results are tabulated in Table 5. The results exhibited that the order of BET surface areas for Fe2O3 products was Fe2O3-400 > Fe2O3-500 > Fe2O3-600. Also, the BJH pore volumes for the Fe2O3 nanostructures were in the same order.
Table 5 BET surface area, pore size, pore volume, and crystallite size of Fe2O3 products and its removal capacity for RR195 dye
| Adsorbent sample |
BET surface area, m2 g−1 |
Crystallite size, nm |
BJH pore diameter (nm) |
BJH pore volume (cm3 g−1) |
Removal adsorption capacity (mg g−1) |
| Fe2O3-400 |
165.6 |
10.5 |
2.24 |
0.385 |
20.5 |
| Fe2O3-500 |
130.3 |
19.8 |
2.02 |
0.266 |
9.6 |
| Fe2O3-600 |
107.7 |
31.2 |
2.24 |
0.202 |
4.7 |
However, as expected, the crystallite size of the aforementioned Fe2O3 products was in the reverse order. Moreover, in order to investigate the influence of the specific surface areas of the iron oxide products on the adsorption process, adsorption capacities of the products toward the RR195 dye were determined under the previously obtained optimum conditions and presented in Table 5. It is clear from this table that Fe2O3-400 adsorbent has the highest adsorption capacity (20.5 mg g−1) and the order of the adsorption capacities of the iron oxide products is in the same order as that for the surface areas. This can be explained based on that the higher surface area provides more contact and exposed sites for dye adsorption and hence this results in higher adsorption capacity.
On the other hand, reusability (i.e. recycling) of the adsorbents for industrial applications purposes is a crucial factor for choosing the suitable adsorbents. Hence, studying the recycling of the Fe2O3-400 adsorbent was performed by regenerating the RR195-loaded adsorbent using either stirring the iron oxide in methanol for 5 h, washing with methanol, and drying at 60 °C for ca. 5 h; or by carrying out combustion of the loaded adsorbent at 500 °C for 30 min. The adsorption of the dye at the optimum conditions and regeneration of the loaded adsorbent was repeated for five times and the percent RR195 dye removal efficiency for the five recycles was depicted in Fig. 18. The results indicate that even after five recycles, Fe2O3-400 adsorbent still has high adsorption efficiency (ca. 94% RR195 dye removal efficiency). The good reusability data suggest that the as-prepared iron oxide adsorbent is an efficient adsorbent and the data also support the long term use of the adsorbent in water treatment from RR195 dye.
 |
| | Fig. 18 RR195 dye removal efficiency of intact and regenerated α-Fe2O3 adsorbent during five adsorption/desorption cycles at 298 K. | |
4. Conclusion
In the present work, pure FeCO3 nanospheres were successfully synthesized in high yield, smaller particle size, and different morphology on comparing to the literatures by hydrothermal reaction of iron sulfate, ascorbic acid as reducing agent, and ammonium carbonate at 140 °C for 1.5 h with molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, respectively. Afterwards, α-Fe2O3 nanoparticles were produced by heating of the as-prepared iron carbonate at 400, 500, or 600 °C for 2 h. The as-prepared products were characterized by means of FE-SEM, XRD, FT-IR, HR-TEM, BET, zeta potential, and thermal analysis. The adsorption properties of the as-prepared hematite nanoparticles were investigated using reactive red 195 (RR195) dye and the results exhibited good adsorption capacity of the nanosized adsorbent. Plus, the α-Fe2O3 nanoparticles produced at 400 °C have the highest adsorption capacity since they have the highest BET surface area. The adsorption data fitted very well with the Langmuir isotherm model indicating that the adsorption reaction was essentially monolayer. The spontaneous and endothermic natures of the adsorption process were confirmed from the thermodynamic studies.
6, respectively. Afterwards, α-Fe2O3 nanoparticles were produced by heating of the as-prepared iron carbonate at 400, 500, or 600 °C for 2 h. The as-prepared products were characterized by means of FE-SEM, XRD, FT-IR, HR-TEM, BET, zeta potential, and thermal analysis. The adsorption properties of the as-prepared hematite nanoparticles were investigated using reactive red 195 (RR195) dye and the results exhibited good adsorption capacity of the nanosized adsorbent. Plus, the α-Fe2O3 nanoparticles produced at 400 °C have the highest adsorption capacity since they have the highest BET surface area. The adsorption data fitted very well with the Langmuir isotherm model indicating that the adsorption reaction was essentially monolayer. The spontaneous and endothermic natures of the adsorption process were confirmed from the thermodynamic studies.
Acknowledgements
The authors thank Benha University, Egypt, for providing the required financial support to perform this research work.
References
- S. Qadri, A. Ganoe and Y. Haik, Removal and recovery of acridine orange from solutions by use of magnetic nanoparticles, J. Hazard. Mater., 2009, 169(1–3), 318–323 CrossRef CAS PubMed.
- B. Tanhaei, A. Ayati, M. Lahtinen and M. Sillanpaa, Preparation and characterization of a novel chitosan/Al2O3/magnetite nanoparticles composite adsorbent for kinetic, thermodynamic and isotherm studies of methyl orange adsorption, Chem. Eng. J., 2015, 259, 1–10 CrossRef CAS.
- F. Qin, G. Li, H. Xiao, Z. Lu, H. Sun and R. Chen, Large-scale synthesis of bismuth hollow nanospheres for highly efficient Cr(VI) removal, Dalton Trans., 2012, 41, 11263–11266 RSC.
- M. Y. Nassar and I. S. Ahmed, A novel synthetic route for magnesium aluminate (MgAl2O4) nanoparticles using sol–gel auto combustion method and their photocatalytic properties, Spectrochim. Acta, Part A, 2014, 131, 320–334 CrossRef PubMed.
- M. Y. Nassar and I. S. Ahmed, Template-free hydrothermal derived cobalt oxide nanopowders: synthesis, characterization, and removal of organic dyes, Mater. Res. Bull., 2012, 47, 2638–2645 CrossRef CAS.
- B. Bi, L. Xu, B. Xu and X. Liu, Heteropoly blue-intercalated layered double hydroxides for cationic dye removal from aqueous media, Appl. Clay Sci., 2011, 54, 242–247 CrossRef CAS.
- M. Khosravi and S. Azizian, Adsorption of anionic dyes from aqueous solution by iron oxide nanospheres, J. Ind. Eng. Chem., 2014, 20, 2561–2567 CrossRef CAS.
- H. Guo, J. Chen, W. Weng, Z. Zheng and D. Wang, Adsorption behavior of Congo red from aqueous solution on La2O3-doped TiO2 nanotubes, J. Ind. Eng. Chem., 2014, 20, 3081–3088 CrossRef CAS.
- M. Y. Nassar, T. Y. Mohamed and I. S. Ahmed, One-pot solvothermal synthesis of novel cobalt salicylaldimine–urea complexes: a new approach to Co3O4 nanoparticles, J. Mol. Struct., 2013, 1050, 81–87 CrossRef CAS.
- M. Y. Nassar, A. S. Attia, K. A. Alfallous and M. F. El-Shahat, Synthesis of two novel dinuclear molybdenum(0) complexes of quinoxaline-2,3-dione: new precursors for preparation of α-MoO3 nanoplates, Inorg. Chim. Acta, 2013, 405, 362–367 CrossRef CAS.
- M. Y. Nassar, Size-controlled synthesis of CoCO3 and Co3O4 nanoparticles by free-surfactant hydrothermal method, Mater. Lett., 2013, 94, 112–115 CrossRef CAS.
- M. Y. Nassar and I. S. Ahmed, Hydrothermal synthesis of cobalt carbonates using different counter ions: an efficient precursor to nano-sized cobalt oxide (Co3O4), Polyhedron, 2011, 30, 2431–2437 CrossRef CAS.
- T. G. Venkatesha, R. Viswanatha, Y. A. Nayaka and B. K. Chethana, Kinetics and thermodynamics of reactive and vat dyes adsorption on MgO nanoparticles, Chem. Eng. J., 2012, 198–199, 1–10 CrossRef CAS.
- H. Emadi and A. N. Kharat, Synthesis and characterization of ultrafine and mesoporous structure of cobalt ferrite, J. Ind. Eng. Chem., 2015, 21, 951–956 CrossRef CAS.
- Z. Zhang and J. Kong, Novel magnetic Fe3O4@C nanoparticles as adsorbents for removal of organic dyes from aqueous solution, J. Hazard. Mater., 2011, 193, 325–329 CrossRef CAS PubMed.
- H. Li, Z. Lu, G. Cheng, K. Rong, F. Chen and R. Chen, HEPES-involved hydrothermal synthesis of Fe3O4 nanoparticles and their biological application, RSC Adv., 2015, 5, 5059–5067 RSC.
- H. Li, Z. Lu, Q. Li, M.-H. So, C.-M. Che and R. Chen, Hydrothermal synthesis and properties of controlled α-Fe2O3 nanostructures in HEPES solution, Chem.–Asian J., 2011, 6, 2320–2331 CrossRef CAS PubMed.
- H. Liang, W. Chen, Y. Yao, Z. Wang and Y. Yang, Hydrothermal synthesis, self-assembly and electrochemical performance of α-Fe2O3 microspheres for lithium ion batteries, Ceram. Int., 2014, 40, 10283–10290 CrossRef CAS.
- A. S. Teja and P. Y. Koh, Synthesis, properties, and applications of magnetic iron oxide nanoparticles, Prog. Cryst. Growth Charact.
Mater., 2009, 55, 22–45 CrossRef CAS.
- M. Khalil, J. Yu, N. Liu and R. L. Lee, Non-aqueous modification of synthesized hematite nanoparticles with oleic acid, Colloids Surf., A, 2014, 453, 7–12 CrossRef CAS.
- M. Mohammadikish, Hydrothermal synthesis, characterization and optical properties of ellipsoid shape α-Fe2O3 nanocrystals, Ceram. Int., 2014, 40, 1351–1358 CrossRef CAS.
- M.-C. Huang, The optical, structural and photoelectrochemical characteristics of porous hematite hollow spheres prepared by DC magnetron sputtering process via polystyrene spheres template, Ceram. Int., 2014, 40, 10537–10544 CrossRef CAS.
- J. Xie, Z. Zhou, Y. Lian, Y. Hao, P. Li and Y. Wei, Synthesis of α-Fe2O3/ZnO composites for photocatalytic degradation of pentachlorophenol under UV-vis light irradiation, Ceram. Int., 2015, 41, 2622–2625 CrossRef CAS.
- M. Gotić, G. Dražić and S. Musić, Hydrothermal synthesis of α-Fe2O3 nanorings with the help of divalent metal cations, Mn2+, Cu2+, Zn2+ and Ni2+, J. Mol. Struct., 2011, 993, 167–176 CrossRef.
- C. Wu, P. Yin, X. Zhu, C. O. Yang and Y. Xie, Synthesis of hematite (α-Fe2O3) nanorods: diameter-size and shape effects on their applications in magnetism, lithium ion battery, and gas sensors, J. Phys. Chem. B, 2006, 110, 17806–17812 CrossRef CAS PubMed.
- S. Rahimi, R. M. Moattaria, L. Rajabia, A. A. Derakhshanb and M. Keyhani, Iron oxide/hydroxide (α,γ-FeOOH) nanoparticles as high potential adsorbents for lead removal from polluted aquatic media, J. Ind. Eng. Chem., 2015, 23, 33–43 CrossRef CAS.
- H. Wu, G. Wu and L. Wang, Peculiar porous α-Fe2O3, γ-Fe2O3 and Fe3O4 nanospheres: facile synthesis and electromagnetic properties, Powder Technol., 2015, 269, 443–451 CrossRef CAS.
- G. Wu, X. Tan, G. Li and C. Hu, Effect of preparation method on the physical and catalytic property of nanocrystalline Fe2O3, J. Alloys Compd., 2010, 504, 371–376 CrossRef CAS.
- A. A. Ayachia, H. Mechakra, M. M. Silvan, S. Boudjaadar and S. Achour, Monodisperse α-Fe2O3 nanoplatelets: synthesis and characterization, Ceram. Int., 2015, 41, 2228–2233 CrossRef.
- T.-W. Sun, Y.-J. Zhu, C. Qi, G.-J. Ding, F. Chen and J. Wu, α-Fe2O3 nanosheet-assembled hierarchical hollow mesoporous microspheres: microwave-assisted solvothermal synthesis and application in photocatalysis, J. Colloid Interface Sci., 2016, 463, 107–117 CrossRef CAS PubMed.
- X.-L. Cheng, J.-S. Jiang, C.-Y. Jin, C.-C. Lin, Y. Zeng and Q.-H. Zhang, Cauliflower-like α-Fe2O3 microstructures: toluene–water interface assisted synthesis, characterization, and applications in wastewater treatment and visible-light photocatalysis, Chem. Eng. J., 2014, 236, 139–148 CrossRef CAS.
- S. Sivakumar, D. Anusuya, C. P. Khatiwada, J. Sivasubramanian, A. Venkatesan and P. Soundhirarajan, Characterizations of diverse mole of pure and Ni-doped α-Fe2O3 synthesized nanoparticles through chemical precipitation route, Spectrochim. Acta, Part A, 2014, 128, 69–75 CrossRef CAS PubMed.
- D. Wang, M. Zhang, J. Yuan, Y. Lin and C. Song, Facile route to Ni-doped α-FeOOH and α-Fe2O3 nanostructures and their properties, Mater. Lett., 2015, 157, 147–150 CrossRef CAS.
- X. J. Liu, H. Wang, C. H. Su, P. W. Zhang and J. B. Bai, Controlled fabrication and characterization of microspherical FeCO3 and α-Fe2O3, J. Colloid Interface Sci., 2010, 351, 427–432 CrossRef CAS PubMed.
- S. Xuan, M. Chen, L. Hao, W. Jiang, X. Gong, Y. Hu and Z. Chen, Preparation and characterization of microsized FeCO3, Fe3O4 and Fe2O3 with ellipsoidal morphology, J. Magn. Magn. Mater., 2008, 320, 164–170 CrossRef CAS.
- T. Yang, Z. Huang, Y. Liu, M. Fang, X. Ouyang and M. Hu, Controlled synthesis of porous FeCO3 microspheres and the conversion to α-Fe2O3 with unconventional morphology, Ceram. Int., 2014, 40, 11975–11983 CrossRef CAS.
- M. Chirita and A. Ieta, FeCO3 microparticle synthesis by Fe–EDTA hydrothermal decomposition, Cryst. Growth Des., 2012, 12, 883–886 CAS.
- R. Jenkins and R. L. Snyder, Chemical Analysis: Introduction to X-ray Powder Diffractometry, John Wiley and Sons, Inc., New York, 1996 Search PubMed.
- K. Nakamoto, Infrared and Raman spectra of inorganic and coordination compounds; Pt. B: Applications in coordination, organometallic, and bioinorganic chemistry, Wiley-Interscience, USA, 5th edn, 1997 Search PubMed.
- K. T. Ehlsissen, A. Delahaya-Vidal, P. Genin, M. Figlarz and P. Willmann, Preparation and characterization of turbostratic Ni/Al layered double hydroxides for nickel hydroxide electrode applications, J. Mater. Chem., 1993, 3, 883–888 RSC.
- R. Suresh, K. Giribabu, R. Manigandan, A. Stephen and V. Narayanan, Fe2O3@polyaniline nanocomposite: characterization and unusual sensing property, Mater. Lett., 2014, 128, 369–372 CrossRef CAS.
- R. A. Bepari, P. Bharali and B. K. Das, Controlled synthesis of α and γ-Fe2O3 nanoparticles via thermolysis of PVA gels and studies on α-Fe2O3 catalyzed styrene epoxidation, J. Saudi Chem. Soc., 2014 DOI:10.1016/j.jscs.2013.12.010.
- M. Ahmaruzzaman and S. L. Gayatri, Batch adsorption of 4-nitrophenol by acid activated jute stick char: equilibrium, kinetic and thermodynamic studies, Chem. Eng. J., 2010, 158, 173–180 CrossRef CAS.
- M. Kosmulski, pH-dependent surface charging and points of zero charge II. Update, J. Colloid Interface Sci., 2004, 275, 214–224 CrossRef CAS PubMed.
- M. Kosmulski, E. Maczka, E. Jartych and J. B. Rosenholm, Synthesis and characterization of goethite and goethite–hematite composite: experimental study and literature survey, Adv. Colloid Interface Sci., 2003, 103, 57–76 CrossRef CAS PubMed.
- E. Liu, H. Zhao, H. Li, G. Li, Y. Liu and R. Chen, Hydrothermal synthesis of porous α-Fe2O3 nanostructures for highly efficient Cr(VI) removal, New J. Chem., 2014, 38, 2911–2916 RSC.
- N. M. Mahmoodi and F. Najafi, Preparation of surface modified zinc oxide nanoparticle with high capacity dye removal ability, Mater. Res. Bull., 2012, 47, 1800–1809 CrossRef CAS.
- M. N. Sepehr, V. Sivasankar, M. Zarrabi and M. S. Kumar, Surface modification of pumice enhancing its fluoride adsorption capacity: an insight into kinetic and thermodynamic studies, Chem. Eng. J., 2013, 228, 192–204 CrossRef CAS.
- Y. S. Ho and G. McKay, Pseudo-second order model for sorption processes, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- W. J. Weber Jr and J. C. Morris, Kinetics of adsorption on carbon from solution, J. Sanit. Eng. Div., Am. Soc. Civ. Eng., 1963, 89, 31–59 Search PubMed.
- C. Luo, Z. Tian, B. Yang, L. Zhang and S. Yan, Manganese dioxide/iron oxide/acid oxidized multi-walled carbon nanotube magnetic nanocomposite for enhanced hexavalent chromium removal, Chem. Eng. J., 2013, 234, 256–265 CrossRef CAS.
- J. Zhou, Z. Zhang, B. Cheng and J. Yu, Glycine-assisted hydrothermal synthesis and adsorption properties of crosslinked porous α-Fe2O3 nanomaterials for p-nitrophenol, Chem. Eng. J., 2012, 211–212, 153–160 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, respectively, at 140 °C for 1.5 h. Pure α-Fe2O3 nanoparticles with an average crystallite size of 10.5–32 nm were produced by thermal decomposition of FeCO3 at 400–600 °C for 2 h. The compositions of the products were identified by means of XRD, FE-SEM, HR-TEM, FT-IR, BET, zeta potential and thermal analysis. The adsorption properties of α-Fe2O3 were evaluated using reactive red 195 (RR195) dye. Various parameters influencing the adsorption process were investigated, using a batch technique. The results show that α-Fe2O3 nanoparticles show good adsorption capacity and the dye removal percentage reaches about 98.77% in 10 min. Plus, increasing the surface area of the α-Fe2O3 nanoparticles from 107.7 to 165.6 m2 g−1 increases the adsorption capacity from 4.7 to 20.5 mg g−1. Moreover, the adsorption data fit the Langmuir isotherm model well and the thermodynamic parameters exhibited an endothermic and spontaneous nature for the adsorption of RR195 dye on the hematite adsorbent.
3, respectively, at 140 °C for 1.5 h. Pure α-Fe2O3 nanoparticles with an average crystallite size of 10.5–32 nm were produced by thermal decomposition of FeCO3 at 400–600 °C for 2 h. The compositions of the products were identified by means of XRD, FE-SEM, HR-TEM, FT-IR, BET, zeta potential and thermal analysis. The adsorption properties of α-Fe2O3 were evaluated using reactive red 195 (RR195) dye. Various parameters influencing the adsorption process were investigated, using a batch technique. The results show that α-Fe2O3 nanoparticles show good adsorption capacity and the dye removal percentage reaches about 98.77% in 10 min. Plus, increasing the surface area of the α-Fe2O3 nanoparticles from 107.7 to 165.6 m2 g−1 increases the adsorption capacity from 4.7 to 20.5 mg g−1. Moreover, the adsorption data fit the Langmuir isotherm model well and the thermodynamic parameters exhibited an endothermic and spontaneous nature for the adsorption of RR195 dye on the hematite adsorbent.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid
ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ammonium carbonate; 1
ammonium carbonate; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) -
-![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 1
3, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) have been investigated.
6) have been investigated.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid
ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO32− molar ratio of 1
CO32− molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1.5 h reaction time, and 140 °C reaction temperature. All the diffraction peaks can be indexed well to a pure rhombohedral phase of FeCO3 which is in consistent with the standard patterns of iron carbonate (space group R
3, 1.5 h reaction time, and 140 °C reaction temperature. All the diffraction peaks can be indexed well to a pure rhombohedral phase of FeCO3 which is in consistent with the standard patterns of iron carbonate (space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c, JCPDS card 83-1764).37 No other characteristic peaks corresponding to iron oxides or even other impurities have been observed. The average crystallite size (D) of the as-prepared FeCO3 was found to be 22 nm which was calculated using the Debye–Scherrer formula:38
c, JCPDS card 83-1764).37 No other characteristic peaks corresponding to iron oxides or even other impurities have been observed. The average crystallite size (D) of the as-prepared FeCO3 was found to be 22 nm which was calculated using the Debye–Scherrer formula:38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θB
θB
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid
ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ammonium carbonate molar ratio using the XRD analysis.
ammonium carbonate molar ratio using the XRD analysis.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 for iron sulfate
6 for iron sulfate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid
ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ammonium carbonate, respectively, for 3 h at different temperatures (160, 140, and 120 °C). It can be noticed that the three temperatures produced iron carbonates and the optimum temperature was 140 °C since it gave pure iron carbonate with a moderate crystallite size (80 nm). However, 160 °C temperature gave pure product with very large crystallite size (200 nm), and 120 °C produced the product with very poor crystallinity contaminated with some impurities as indicated from their XRD patterns and appearance of some peaks for some undefined by-products.
ammonium carbonate, respectively, for 3 h at different temperatures (160, 140, and 120 °C). It can be noticed that the three temperatures produced iron carbonates and the optimum temperature was 140 °C since it gave pure iron carbonate with a moderate crystallite size (80 nm). However, 160 °C temperature gave pure product with very large crystallite size (200 nm), and 120 °C produced the product with very poor crystallinity contaminated with some impurities as indicated from their XRD patterns and appearance of some peaks for some undefined by-products.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid
ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ammonium carbonate molar ratio of 1
ammonium carbonate molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. The results indicated that the optimum reaction time was 1.5 h. On the other hand, shorter time (i.e. <1.5 h) produced iron carbonate with poor crystallinity and low yield, but longer reaction time gave particles with very large crystallite size. On comparing the optimum temperature and time in the current study with other reported ones for preparing pure iron carbonate, it can easily be seen that we have used lower temperature and shorter time than the reported ones.34–36
6. The results indicated that the optimum reaction time was 1.5 h. On the other hand, shorter time (i.e. <1.5 h) produced iron carbonate with poor crystallinity and low yield, but longer reaction time gave particles with very large crystallite size. On comparing the optimum temperature and time in the current study with other reported ones for preparing pure iron carbonate, it can easily be seen that we have used lower temperature and shorter time than the reported ones.34–36
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO32− molar ratio of 1
CO32− molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 in absence of ascorbic acid, and the XRD pattern of the product is shown in Fig. 4(a). Also, the influence of addition of different quantities of ascorbic acid have been explored by trying different Fe2+
6 in absence of ascorbic acid, and the XRD pattern of the product is shown in Fig. 4(a). Also, the influence of addition of different quantities of ascorbic acid have been explored by trying different Fe2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid
ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO32− molar ratios: 1
CO32− molar ratios: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, and 1
6, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6; and the XRD results are depicted in Fig. 4(b)–(e), respectively. Inspection of XRD patterns of the produced products (Fig. 4) reveals that the minimum required equivalents of ascorbic acid are 1 (i.e. Fe2+
6; and the XRD results are depicted in Fig. 4(b)–(e), respectively. Inspection of XRD patterns of the produced products (Fig. 4) reveals that the minimum required equivalents of ascorbic acid are 1 (i.e. Fe2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid
ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO32− optimum molar ratio is 1
CO32− optimum molar ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) and using less equivalents of the ascorbic acid (i.e. <1 equivalent) gives iron carbonate with poor crystallinity along with some impurities such as magnetite. It is worthy to mention that in the current hydrothermal treatment smaller quantity of ascorbic acid has been used to produce pure iron carbonate with smaller particle size on comparing to the other reported methods.34–36 However, the mechanism of the current hydrothermal process can be explained according to the proposed Scheme 1 as follows: ammonium carbonate and iron sulfate generate carbonate and iron ions according to reactions (i) and (ii), respectively. At the optimum or higher concentrations of both carbonate ions and ascorbic acid, reactions (iii) and (vii) (Scheme 1) will be the more predominant reactions and accordingly the product will be pure iron carbonate. At lower concentration of ascorbic acid, Fe3+ ions (produced from oxidation of Fe2+ during mixing or heating) will not be completely reduced to Fe2+ ions (reaction (iv)) and in turn Fe3+ ions will react with OH− (which can be generated from reaction (v)) to produce Fe(OH)3. Thence, there will be a competition between reactions (iii), (vii) and (ix) and the result of that competition will be a mixture of iron carbonate and Fe3O4. Additionally, Fe3O4 impurities are by-products generated from the reaction between Fe(OH)3 (reaction (viii)) and Fe(OH)2 (reaction (vi)) at the hydrothermal conditions according to reaction (ix) (Scheme 1) and this is in agreement with the reported data.36
6) and using less equivalents of the ascorbic acid (i.e. <1 equivalent) gives iron carbonate with poor crystallinity along with some impurities such as magnetite. It is worthy to mention that in the current hydrothermal treatment smaller quantity of ascorbic acid has been used to produce pure iron carbonate with smaller particle size on comparing to the other reported methods.34–36 However, the mechanism of the current hydrothermal process can be explained according to the proposed Scheme 1 as follows: ammonium carbonate and iron sulfate generate carbonate and iron ions according to reactions (i) and (ii), respectively. At the optimum or higher concentrations of both carbonate ions and ascorbic acid, reactions (iii) and (vii) (Scheme 1) will be the more predominant reactions and accordingly the product will be pure iron carbonate. At lower concentration of ascorbic acid, Fe3+ ions (produced from oxidation of Fe2+ during mixing or heating) will not be completely reduced to Fe2+ ions (reaction (iv)) and in turn Fe3+ ions will react with OH− (which can be generated from reaction (v)) to produce Fe(OH)3. Thence, there will be a competition between reactions (iii), (vii) and (ix) and the result of that competition will be a mixture of iron carbonate and Fe3O4. Additionally, Fe3O4 impurities are by-products generated from the reaction between Fe(OH)3 (reaction (viii)) and Fe(OH)2 (reaction (vi)) at the hydrothermal conditions according to reaction (ix) (Scheme 1) and this is in agreement with the reported data.36
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) CO32− molar ratios. Effect of ammonium carbonate concentration has been investigated by keeping Fe2+ ion and ascorbic acid concentrations constant and varying the concentration of ammonium carbonate by using the following different molar ratios: 1
CO32− molar ratios. Effect of ammonium carbonate concentration has been investigated by keeping Fe2+ ion and ascorbic acid concentrations constant and varying the concentration of ammonium carbonate by using the following different molar ratios: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 1
3, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 for Fe2+
6 for Fe2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid
ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO32−, respectively, and the XRD patterns of the products are given in Fig. 5(a)–(d), respectively. The obtained results reveal that using lower concentration of CO32− anion than 3 equivalents produces by-products along with FeCO3 with very poor crystallinity. Consequently, it can be concluded that the optimum molar ratio for this template-free hydrothermal reaction is (1
CO32−, respectively, and the XRD patterns of the products are given in Fig. 5(a)–(d), respectively. The obtained results reveal that using lower concentration of CO32− anion than 3 equivalents produces by-products along with FeCO3 with very poor crystallinity. Consequently, it can be concluded that the optimum molar ratio for this template-free hydrothermal reaction is (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) for Fe2+
3) for Fe2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid
ascorbic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO32−, respectively.
CO32−, respectively.
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c, lattice constant; a = 5.038 Å and c = 13.776 Å, JCPDS card 89-0598). The estimated average crystallite size of the produced α-Fe2O3 nanoparticles using the Debye–Scherrer equation38 was found to be ca. 10.5 nm. Moreover, the as-prepared FeCO3 precursor was also calcinated at 500 and 600 °C to α-Fe2O3 nanoparticles with different crystallite sizes; 19.8 and 31.2 nm, respectively, calculated from the XRD patterns of the produced α-Fe2O3, Fig. 1(c) and (d), using the Debye–Scherrer equation. It is noticeable that higher calcination temperatures produced larger crystallite sizes. The surface morphology and microstructure of α-Fe2O3 particles produced at 600 °C and displayed in Fig. 6(II) and 9 have been investigated using FE-SEM and TEM, respectively. It can be seen from Fig. 6(II) that α-Fe2O3 product is clusters of individually spherical shape α-Fe2O3 particles and this indicates that iron carbonate morphologies on calcination have almost been retained for α-Fe2O3 product. Inspection the size and morphology of the α-Fe2O3 particles, Fig. 9, using TEM images exhibits that the α-Fe2O3 product consists of irregular and spherical morphology with an average particle size of ca. 32 nm which is consistent with the one obtained using XRD data. Chemical structure of α-Fe2O3 produced at 600 °C (Fe2O3-600 product) was also confirmed by using Fourier transform infrared spectrum, Fig. 7(b), which exhibited two strong absorption bands at 547.7 and 461.9 cm−1 corresponding to the Fe–O bond which is consistent with the published data.21,41,42 Plus, the two bands appeared at 3370 and 1600 cm−1 can be attributed to the stretching and bending vibrations of adsorbed surface water molecules interacting with the oxide product and the broadness of these peaks may be assigned to hydrogen bonding O–H.39
c, lattice constant; a = 5.038 Å and c = 13.776 Å, JCPDS card 89-0598). The estimated average crystallite size of the produced α-Fe2O3 nanoparticles using the Debye–Scherrer equation38 was found to be ca. 10.5 nm. Moreover, the as-prepared FeCO3 precursor was also calcinated at 500 and 600 °C to α-Fe2O3 nanoparticles with different crystallite sizes; 19.8 and 31.2 nm, respectively, calculated from the XRD patterns of the produced α-Fe2O3, Fig. 1(c) and (d), using the Debye–Scherrer equation. It is noticeable that higher calcination temperatures produced larger crystallite sizes. The surface morphology and microstructure of α-Fe2O3 particles produced at 600 °C and displayed in Fig. 6(II) and 9 have been investigated using FE-SEM and TEM, respectively. It can be seen from Fig. 6(II) that α-Fe2O3 product is clusters of individually spherical shape α-Fe2O3 particles and this indicates that iron carbonate morphologies on calcination have almost been retained for α-Fe2O3 product. Inspection the size and morphology of the α-Fe2O3 particles, Fig. 9, using TEM images exhibits that the α-Fe2O3 product consists of irregular and spherical morphology with an average particle size of ca. 32 nm which is consistent with the one obtained using XRD data. Chemical structure of α-Fe2O3 produced at 600 °C (Fe2O3-600 product) was also confirmed by using Fourier transform infrared spectrum, Fig. 7(b), which exhibited two strong absorption bands at 547.7 and 461.9 cm−1 corresponding to the Fe–O bond which is consistent with the published data.21,41,42 Plus, the two bands appeared at 3370 and 1600 cm−1 can be attributed to the stretching and bending vibrations of adsorbed surface water molecules interacting with the oxide product and the broadness of these peaks may be assigned to hydrogen bonding O–H.39





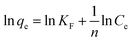
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe against ln
qe against ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce (Fig. 16(b)). The Langmuir and Freundlich constants are presented in Table 3. Clearly, the adsorption of RR195 dye on the α-Fe2O3 adsorbent follows the Langmuir adsorption isotherm model and the correlation coefficient for this model is estimated to be 0.992. Plus, fitting of the experimental data to Langmuir isotherm model exhibits the homogeneous nature of α-Fe2O3 surface which in turn means that each RR195 dye molecule/adsorption has equal activation energy of adsorption. Hence, the results reveal that the adsorption of the RR195 dye is monolayer coverage at the outer surface of α-Fe2O3 adsorbent. Moreover, the efficiency of the adsorption of the RR195 dye on the adsorbent can be estimated through the values of the separation factor constant (RL) which can be given as follows:
Ce (Fig. 16(b)). The Langmuir and Freundlich constants are presented in Table 3. Clearly, the adsorption of RR195 dye on the α-Fe2O3 adsorbent follows the Langmuir adsorption isotherm model and the correlation coefficient for this model is estimated to be 0.992. Plus, fitting of the experimental data to Langmuir isotherm model exhibits the homogeneous nature of α-Fe2O3 surface which in turn means that each RR195 dye molecule/adsorption has equal activation energy of adsorption. Hence, the results reveal that the adsorption of the RR195 dye is monolayer coverage at the outer surface of α-Fe2O3 adsorbent. Moreover, the efficiency of the adsorption of the RR195 dye on the adsorbent can be estimated through the values of the separation factor constant (RL) which can be given as follows:


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc
Kc
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc against 1/T gives a straight line (Fig. 17). ΔS0 and ΔH0 are calculated from the slope and intercept, and presented in Table 4. Inspection of Table 4 reveals the endothermic nature of the adsorption process as indicated from the positive value of the ΔH0. Plus, the negative value of the ΔG0 means that the adsorption of RR195 dye on the hematite nanoparticles is a spontaneous nature and the decrease in ΔG0 values with increasing the temperature indicates that the adsorption process is more preferable at higher temperatures.
Kc against 1/T gives a straight line (Fig. 17). ΔS0 and ΔH0 are calculated from the slope and intercept, and presented in Table 4. Inspection of Table 4 reveals the endothermic nature of the adsorption process as indicated from the positive value of the ΔH0. Plus, the negative value of the ΔG0 means that the adsorption of RR195 dye on the hematite nanoparticles is a spontaneous nature and the decrease in ΔG0 values with increasing the temperature indicates that the adsorption process is more preferable at higher temperatures.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc
Kc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, respectively. Afterwards, α-Fe2O3 nanoparticles were produced by heating of the as-prepared iron carbonate at 400, 500, or 600 °C for 2 h. The as-prepared products were characterized by means of FE-SEM, XRD, FT-IR, HR-TEM, BET, zeta potential, and thermal analysis. The adsorption properties of the as-prepared hematite nanoparticles were investigated using reactive red 195 (RR195) dye and the results exhibited good adsorption capacity of the nanosized adsorbent. Plus, the α-Fe2O3 nanoparticles produced at 400 °C have the highest adsorption capacity since they have the highest BET surface area. The adsorption data fitted very well with the Langmuir isotherm model indicating that the adsorption reaction was essentially monolayer. The spontaneous and endothermic natures of the adsorption process were confirmed from the thermodynamic studies.
6, respectively. Afterwards, α-Fe2O3 nanoparticles were produced by heating of the as-prepared iron carbonate at 400, 500, or 600 °C for 2 h. The as-prepared products were characterized by means of FE-SEM, XRD, FT-IR, HR-TEM, BET, zeta potential, and thermal analysis. The adsorption properties of the as-prepared hematite nanoparticles were investigated using reactive red 195 (RR195) dye and the results exhibited good adsorption capacity of the nanosized adsorbent. Plus, the α-Fe2O3 nanoparticles produced at 400 °C have the highest adsorption capacity since they have the highest BET surface area. The adsorption data fitted very well with the Langmuir isotherm model indicating that the adsorption reaction was essentially monolayer. The spontaneous and endothermic natures of the adsorption process were confirmed from the thermodynamic studies.
















