Fabrication of monolithic carbon nanofiber/carbon composites†
Abstract
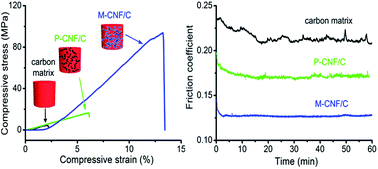
Monolithic carbon nanofiber/carbon (M-CNF/C) composites were fabricated through facile liquid impregnation and hot pressing technologies, using monolithic carbon nanofibers and phenolic resin as reinforcement and carbon matrix precursor, respectively. The M-CNFs are uniformly dispersed in the M-CNF/C composites and display strong interfacial bonding with carbon matrix. Compared with powdered carbon nanofiber reinforced (P-CNF/C) composites, M-CNF/C composites exhibit significantly higher improvement in electrical conductivity, thermal conductivity and mechanical properties. The M-CNF/C composites also exhibit much lower friction coefficients (0.09–0.12) and wear losses (0.12–0.43 mg) than those of P-CNF/C composites. The superior enhancement is attributed to the unique 3D interconnected structure and high integrity of M-CNFs, which could dramatically increase conductive channels, significantly improve mechanical properties and remarkably decrease friction coefficient and wear loss of M-CNF/C composites. Furthermore, the electrical, thermal, mechanical and tribological properties of M-CNF/C composites could be adjusted by using M-CNFs with different bulk densities. The present work suggests the M-CNF/C composites a widespread potential as high-performance tribological materials.

 Please wait while we load your content...
Please wait while we load your content...