Investigation of Ce(III) promoter effects on the tri-metallic Pt, Pd, Ni/MgO catalyst in dry-reforming of methane
Faris A. J. Al-Doghachiab,
Umer Rashidc and
Yun Hin Taufiq-Yap*ab
aCatalysis Science and Technology Research Centre, Faculty of Science, University Putra Malaysia, 43400 UPM, Serdang, Selangor, Malaysia. E-mail: taufiq@upm.edu.my; Fax: +60-3-89466758; Tel: +60-3-89466809
bDepartment of Chemistry, Faculty of Science, University Putra Malaysia, 43400 UPM, Serdang, Selangor, Malaysia
cInstitute of Advanced Technology, University Putra Malaysia, 43400 UPM, Serdang, Selangor, Malaysia
First published on 19th January 2016
Abstract
A mixture of cerium oxide and magnesium oxide supports with certain mole ratios of Mg2+/Ce3+ were prepared via the co-precipitation of Mg and Ce nitrates, and followed by impregnation with 1 wt% each of Ni, Pd, and Pt metals to form Pt, Pd, Ni/Mg1−xCexO catalysts. Evaluation of the prepared catalysts was carried out by a DRM reaction for 200 h and they were characterised by means of in situ XRD, XRF, XPS, BET, H2-TPR, TEM and TGA. It was found that the interaction of a suitable amount of MgO with Ce2O3 stabilised a cubic phase in the catalysts, which has a high basicity to adsorb CO2 forming a monoclinic Ce2O2CO3 species in the DRM reaction. The introduction of MgO also created surface oxygen ions. The oxidisation and the removal of the deposited carbon maybe achieved by both monoclinic Ce2O2CO3 and surface oxygen, keeping the metal Ni, Pd, and Pt catalyst at high activity and stability. The Ce2O3 as a promoter in the catalyst had several effects such as: stabilisation of the magnesia cubic phase; increase in its thermal stability, increase in the basicity of the support, decrease in the carbon deposition, and decrease in the reducibility of the Ni2+, Pd2+, and Pt2+ ions.
1. Introduction
The demand for alternative energy resources has recently been on the rise due to the fast depletion of fossil fuel. Therefore, the utilisation of greenhouse gases, such as methane and carbon dioxide, as alternative energy has received much attention. It has been found that the reserves of methane are larger than crude oil reserves because methane can be produced from various sources, including shale gas, fermented wastes, and methane hydrates.1 Methane can be transformed into synthesis gas (a mixture of H2 and carbon CO, also known as syngas) through reforming reactions using steam, partial oxidation or carbon dioxide.The synthesis gas is an important feedstock for fuels and/or the production of chemicals in industries. Coal, petroleum, natural gas, and biomass can be converted into syngas. However, varying the H2/CO molar ratios is required in accordance with the industrial applications of syngas. For example, the H2/CO ratio of 2 is required for methanol synthesis;2 whilst the ratio is controlled at 1 for dimethyl ether (DME) synthesis under the single step process.3 In the Fisher–Tropsch process, the H2/CO ratio is usually in the range of 1 to 2, depending on the type of fuel synthesized.4 Consequently, the H2/CO ratio in syngas plays an important role in fuel production. Recently, a number of studies5 that have been carried out have focused on the indirect reduction of iron oxides using H2 and CO as reducing agents. The indirect reduction of iron oxides involves two mechanisms: CO-based and H2-based.6 The two gases or syngas can be produced from the reforming of coke oven gas (COG). In this aspect, there is no limit of the H2/CO ratio in the utilisation of syngas for the iron making processes, whereby H2 and CO are able to individually trigger the indirect reduction in a blast furnace.
The conversion of CH4 to the synthesis gas, which constitutes the feed for the Fischer–Tropsch syntheses, is very important from an industrial point of view. The dominant commercial method employed to produce synthesis gas is the steam reforming of methane (eqn (1)).7
| CH4 + H2O → CO + 3H2 ΔH°298K = 225.0 kJ mol−1 | (1) |
However, there are several limitations to this reaction. First, the energy consumption for this reaction is higher. Next, the selectivity for CO for this reaction is poor. Finally, the reaction is unsuitable for Fischer–Tropsch syntheses because its H2/CO ratio is high. As a result, many researchers have attempted to convert methane to syngas through the catalytic partial oxidation of methane (eqn (2)).8
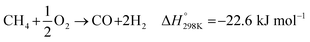 | (2) |
Although this conversion is only mildly exothermic, a small decrease in the selectivity of CO due to the total combustion of methane, which is a highly exothermic reaction, has resulted in a large increase in the reaction temperature. Furthermore, a high methane conversion together with a high space velocity can produce a large amount of heat in a small region of the catalyst. As it is difficult to remove this heat from the reactor, especially in equipment for large-scale purposes, the process can become difficult to control.9
Several researchers have proposed to reform methane catalytically with CO2 instead of steam, to obtain high selectivity of CO and more appropriate H2/CO ratios. They conducted the dry reforming of methane (DRM) (eqn (3)).10
| CH4 + CO2 → 2CO + 2H2, ΔH°298 K = 247.0 kJ mol−1 | (3) |
This reaction bears important environmental implications for both the greenhouse gases, methane and carbon dioxide, which can be converted into valuable feedstocks.
The main setback of the DRM reaction is coke formation, which is caused by methane decomposition (eqn (4)) and the Boudouard reaction (eqn (5)). However, it has been reported that nickel-based catalysts can be suppressed by adding promoters in the deactivation process. In fact, strong Lewis bases (e.g., MgO, CaO) that are enhanced with the chemisorb of CO2, have resulted in the reduction of coke deposition in the reaction with C to form CO. Similar effects have been reported for lanthanide elements, such as CeO2 and La2O3, that can store and release oxygen, leading to the removal of carbon in the reaction between the deposited carbon and the lattice oxygen formed in these redox oxides.11
| CH4 → C + 2H2, ΔH°298 K = 75.0 kJ mol−1 | (4) |
| 2CO → C + CO2, ΔH°298K = −172.0 kJ mol−1 | (5) |
The metal, nickel, is more commonly used as the active metal in the reforming process as its relatively abundant and its cost is low. However, the only drawback is that nickel easily induces the formation of carbon leading to catalytic deactivation.12 As a result, numerous research studies have been carried out to improve the catalytic activity and stability of nickel-based catalysts in the reforming process.13 The deactivation of nickel-based catalysts can be suppressed by the addition of promoters, such as strong Lewis bases (e.g., MgO, CaO) enhanced with the chemisorb of CO2, in order to reduce coke deposition in the reaction with C to form CO.14
It is noted that noble metals, such as Pt, Rh and Ru, are highly active towards DRM. Also, they are more resistant to the formation of carbon in comparison to other transition metals,15 Also, promoting Ni catalysts with noble metals that include Rh, Pt, Pd or Ru adds to the activity of the catalysts that show more stability against coke deposition when compared to the other non-promoted Ni catalysts.16 For example, bimetallic Ni–Pt that supports ZrO2 shows higher and more stable activity for a prolonged period of time than mono-metallic Ni/ZrO2. Hence, Ni–Pt shows potential in the industrial application for DRM.17 Meanwhilst, the activity and stability of bimetallic Ni–Pd catalysts are much higher than the mono-metallic Ni catalyst.18 These findings are consistent with the hypothesis that Pd helps in the prevention of the oxidation of Ni. A recent study over the promotion of Ni/MgO with Sn, Ce, Mn and Co reported higher catalytic activity and stability for Co-promoted catalysts; moreover, the coke deposition resistance was high for Sn and Co promoted catalysts. The higher catalytic activity for Co-promoted catalysts can be attributed to its high affinity for oxygen species enhancing it coke resistance properties. While Ce and Mn promoted catalysts exhibited lower catalytic performance, for Mn-promoted catalyst the agglomeration was the sole reason for its low catalytic activity and for Ce promoted catalyst lower activity was observed due to segregation of Ce as CeO2 due to its immiscibility with MgO which lead to the larger Ni0 particles.19
As such, the objective of this study has been to prepare a catalyst with high activity, selectivity, stability as well as the ability to prevent carbon deposition on the catalyst during the dry reforming of the methane reaction. The Pt, Pd, Ni/Mg1−xCexO catalysts were prepared by using the co-precipitation method that uses K2CO3 as a precipitant, followed by the impregnation of 1% Pt, 1% Pd, and 1% Ni using Pt(acac)2, Pd(acac)2, and Ni(acac)2, respectively. This was followed by a study into the comparison between the catalytic stability and coke formation. In addition, the study was also investigated the effects of the concentrations of CO2 and CH4, concentration of the catalysts, and the temperature of the conversion of the catalytic performance of the prepared catalysts in the dry reforming process as well as the study evaluated the stability of the catalyst. Finally, the study was examined the enhancement of the methane conversion of a stream of 1.25% oxygen gas passing through the reaction.
2. Experimental
2.1 Support and catalyst preparations
The catalysts, Mg1−xCexO (x = 0.00, 0.03, 0.07, 0.15), were prepared using the co-precipitation method as reported previously.20 Meanwhilst, the support MgO and promoter ceria Ce2O3 were prepared using a 0.1 M aqueous solution of Ce(NO3)3·6H2O (Merck; >99.0%) and Mg(NO3)2·6H2O (Merck; >99.0%) as the amount in the Table 1, and 1 M K2CO3 (Merck; >99.7%), which were used as the precipitants. The sample was washed with hot water after the filtration of the precipitant. Next, the sample was dried at 120 °C for 12 hours. Subsequently, it was pre-calcined in air at 500 °C for 5 h to remove CO2 from the precipitant. After that, the sample was pressed into disks at 600 kg m−2, and then calcined at 1150 °C for 20 h for enhancement of the mechanical properties and for ensuring good interaction between the support MgO and the promoter Ce2O3.| Catalysts | Support (MgO) Mg(NO3)2·6H2O (g) | Promoter (Ce2O3) Ce(NO3)3·6H2O (g) | Total weight of MgO and Ce2O3 after calcine (g) | Impregnation of the main catalyst (1% Pt) (1% Pd) (1% Ni) (g) | ||
|---|---|---|---|---|---|---|
| Pt(acac)2 | Pd(acac)2 | Ni(acac)2 | ||||
| Pt/MgO | 25.0 | 0.0 | 1 | 0.02 | 0.029 | 0.044 |
| Pt, Pd, Ni/Mg0.97Ce0.033+O | 24.9 | 1.3 | 1 | 0.02 | 0.029 | 0.044 |
| Pt, Pd, Ni/Mg0.93Ce0.073+O | 23.8 | 3.0 | 1 | 0.02 | 0.029 | 0.044 |
| Pt, Pd, Ni/Mg0.85Ce0.153+O | 21.8 | 6.5 | 1 | 0.02 | 0.029 | 0.044 |
Table 1 shows the preparation of the catalyst. First, 1% Pt was impregnated using Pt(C5H7O2)2. H2O (Acros Chemicals; >99%) which was dissolved with dichloromethane for 5 h to produce Pt(acac)2/Mg1−xCexO. Finally, the catalysts, Pt, Pd, Ni(acac)2/Mg1−xCexO, were prepared by impregnating Pt(acac)2/Mg1−xCexO with a 1% of each Pd and Ni by using a solution of Pd(C5H7O2)2 (Aldrich; >99.5%) and Ni(C5H7O2)2·H2O (Acros Chemicals; >99%) in dichloromethane for 5 h, respectively. After impregnation in the air, the catalysts were dried for 12 h at a temperature of 120 °C. The dried catalysts were crushed and sieved to particles with 80–150 or 150–250 μm diameter.
2.2 Catalyst characterisation
The thermogravimetric analysis (TGA) was carried out on a Mettler Toledo TG-DTA Apparatus (Pt crucibles, Pt/Pt–Rh thermocouple) with the purge gas (nitrogen) flow rate of 30 ml min−1 and the heating rate of 10 °C min−1 from 50 to 1000 °C.An X-ray diffraction analysis was performed using a Shimadzu diffractometer model XRD 6000. The diffractometer employed Cu-Kα radiation to generate diffraction patterns from powder crystalline samples at an ambient temperature. The Cu-Kα radiation was generated by Philips glass diffraction X-ray tube broad focus 2.7 kW type. The crystallite size D of the samples was calculated using the Debye–Scherrer's relationship.21 Where D was the crystalline size, λ was the incident X-ray wavelength, β was the full width at half-maximum (FWHM), and θ was the diffraction angle.
The Fourier transform infrared (FT-IR) analysis was carried out with the PerkinElmer spectrometer model 100 series (sample preparation UATR).
The total catalyst surface area was obtained using the Brunauer–Emmett–Teller (BET) method with nitrogen adsorption at −196 °C. The analysis was conducted using a Thermo Fisher Scientific S.P.A (model: Surfer Analyser) nitrogen adsorption–desorption analyser.
Transmission electron microscopy (TEM) (Hitachi H7100 TEM with accelerating voltage of 10 MV) was used to determine the crystal shape and the homogeneity of the catalysts.
Field Emission Scanning Electron Microscopy (FE-SEM) was used. The sample morphology was studied with the JEOL Field Emission scanning Electron Microscope (FE-SEM) model JSM 7600F at a very high magnification by using the field emission current. The particles of the samples were glued onto an aluminium stud by using double-sided tape. Then, it was coated with gold to make sure of the better visibility of the surface and to prevent electrical charging of the sample during analysis.
The active site of the catalysts was evaluated by Temperature Programmed Reduction (H2-TPR) using hydrogen conducted by Thermo Finnegan TPDRO 1100 apparatus Equipped with a thermal conductivity detector. In the reactor, about 0.05 g of the catalyst was placed and treated under 150 °C for 30 min in N2 (20 ml min−1). The analysis of hydrogen 5.51% in argon was carried out between 50 °C and 950 °C under argon flow (10 °C min−1, 25 ml min−1) and detected by a thermal conductivity detector.
XPS spectra were obtained using the Kratos Axis Ultra DLD system, equipped with a monochromatic Al Kα (1486.6 eV), dual X-ray sources (Al & Mg), an argon etching system for sample cleaning and depth profiling, parallel imaging XPS, AES, ISS and Vision software for controlling the system. The base pressure of the analyser chamber was 1 × 10−10 Torr. The excitation source, X-ray gun, was operated as a combination of 20 mA of emission current and 15 kV voltages. The hemispherical analyser was operated in the fixed analyser transmission (FAT) mode for both wide and narrow scanning. This value was set at 100 eV and 40 eV of the pass energy, respectively. The region of interest for the narrow scan corresponded to Mg2p, Ce3d, Pd3d, Ni2d, Pt4f and O1s photoelectron signals. The carbon charging correction refers to the binding energy of adventitious carbon at the binding energy of 285 eV. This highly sophisticated equipment is considered as a non-destructive analysis technique due to soft X-ray production to induce photoelectron emission from the sample surface. Therefore, the equipment was able to provide information about the surface layers or thin film structures (about the top 10–100 Å of the sample).
2.3 Catalytic evaluations
The catalytic evaluation for dry reforming of methane with CO2 (DRM) towards syngas (H2/CO) production as the model biogas reforming was carried out using a fixed bed stainless steel micro-reactor (i.d. Ø = 6 mm, h = 34 cm). The reactor was connected to a mass flow gas controller (SIERRA instrument) and an online gas chromatography (GC) (Agilent 6890N; G 1540N) equipped with Varian capillary columns HP-PLOT/Q and HP-MOLSIV. Prior to the reaction, approximately 0.02 g of the catalyst was reduced by flowing 5% H2/Ar (30 ml min−1) at 700 °C and holding for 3 h. The reforming reaction was performed by flowing the feed, a gas mixture consisting of CH4/CO2 in (2/1) and (1/1) mol, at a rate of 30 ml min−1. The reforming has been studied from 700 to 900 °C at 1 atm, then holding was carried out for 10 h (1 atm, GHSV = 30 ml min−1).The reduction step was aimed to reduce the (Ni2+, Pd2+, Pt2+) phase of the catalyst to the metals (Ni0, Pd0, Pt0) phase at the active site of the catalysts. The tested catalyst was placed in the middle of a reactor vertically and held in place by plugs of quartz wool. In order to control and ensure the reaction temperature, a thermocouple was placed into the catalyst chamber. The calculations for the CH4 and CO2 conversions, H2 and CO selectivity, as well as syngas (H2/CO) ration were defined as the following equations (eqn (6)–(10)).
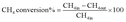 | (6) |
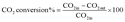 | (7) |
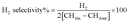 | (8) |
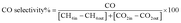 | (9) |
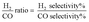 | (10) |
3. Results and discussion
3.1. Characterisation of the catalysts
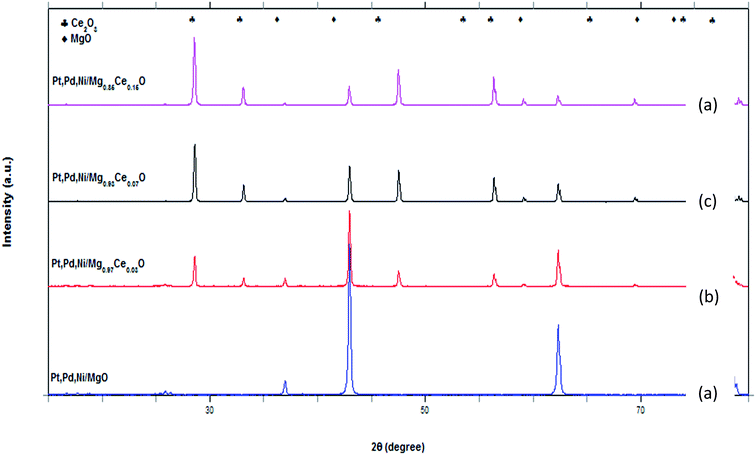 | ||
| Fig. 1 XRD patterns of the catalysts (a) Pt, Pd, Ni/MgO (b) Pt, Pd, Ni/Mg0.97Ce0.033+O (c) Pt, Pd, Ni/Mg0.93Ce0.073+O (d) Pt, Pd, Ni/Mg0.85Ce0.153+O. | ||
| Catalysts | TEM (nm) | Crystal size (D) Debye Sherrer eq. (nm) | Ni% | Pd% | Pt% | Mg & Ce% |
|---|---|---|---|---|---|---|
| Pt, Pd, Ni/MgO | 56 | 42.2 | 1.13 | 1.09 | 1.15 | 96.30 |
| Pt, Pd, Ni/Mg0.97Ce0.033+O | 68 | 53.3 | 1.18 | 1.11 | 1.19 | 96.17 |
| Pt, Pd, Ni/Mg0.93Ce0.073+O | 59 | 48.7 | 1.07 | 1.10 | 1.15 | 96.2 |
| Pt, Pd, Ni/Mg0.85Ce0.153+O | 61 | 48.0 | 1.13 | 1.22 | 1.26 | 96.1 |
For the elemental analysis of all the components in the catalyst, XRF has been used. Table 2 shows that the Ni, Pd, and Pt percentages were slightly more than 1, which was due to the incomplete precipitation of the magnesium and cerium metal precursors in the method of co-precipitation. This had a slight effect on the results.24
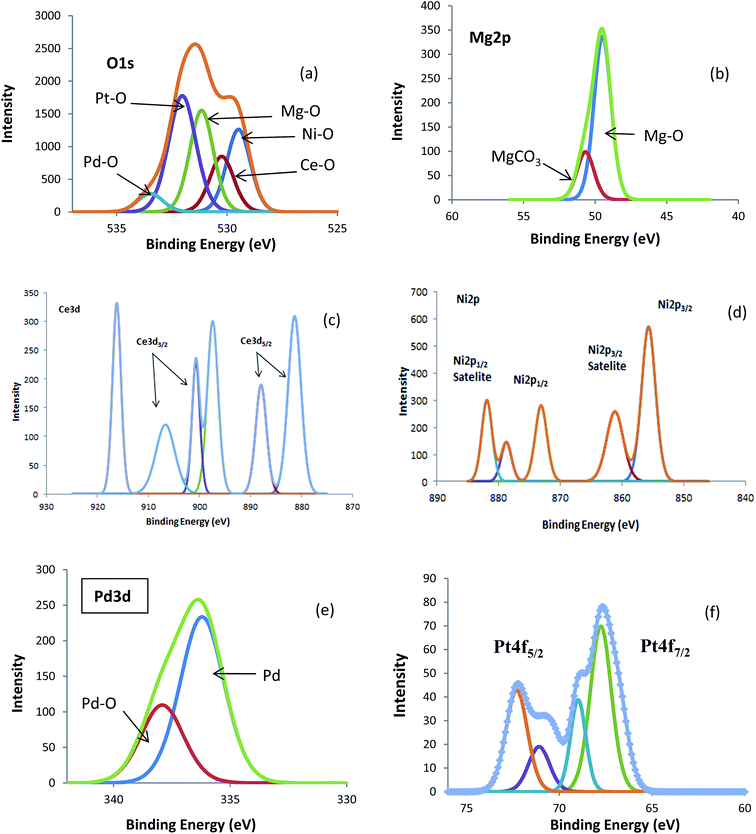 | ||
| Fig. 2 XPS narrow scans of the reduced catalyst. (a) O1s (b) Mg2p (c) Ce3d (d) Ni2p (e) Pd3d (f) Pt4f. | ||
 | ||
| Fig. 3 TPR-H2 profiles of catalysts reduced in a (5% H2/Ar) stream at a temperature ramp of 10 °C min−1. | ||
The analysis of XPS indicates that the solid solution of Ce2O3–MgO shows a low and a high binding energy of 51 eV and 882.5 eV for Mg2p and Ce3d, respectively. As a result, it is imperative to transfer the electrons from Ce2O3 to MgO. This is to slow down the reduction of Ce2O3 during the preparation of the reduced catalyst. However, during the slowdown, there is an increase in the interaction between the two oxides. This leads to the segregation of Ce atoms as small particles on the surface of the catalyst, resulting in a high dispersion of Ce, which is responsible for the high level of activity of the catalyst. Furthermore, the segregated Ce particles, which have been extracted from the substrate, interact strongly with the Ce remaining on the substrate, resulting in the attenuation of their sintering. In this case, there is no formation of coke due to the high dispersion of Ce on the surface of the catalyst. Also, the Ce clusters are not big enough for the formation of coke. The TEM has indicated that a highly effective Ce2O3–MgO solid solution contains crystallite sizes of about 80 nm.18
| Catalysts | Temp. °C | Temp. °C | Temp. °C | Temp. °C | Temp. °C | Amount H2 gas adsorbed (μmol g−1) |
|---|---|---|---|---|---|---|
| Pt, Pd, Ni/MgO | 130 | 184 | 511 | — | — | 488.6 |
| Pt, Pd, Ni/Mg0.97Ce0.033+O | 129 | 169 | 481 | 502 | 711 | 429.1 |
| Pt, Pd, Ni/Mg0.93Ce0.073+O | 132 | 198 | 495 | 508 | 727 | 941.9 |
| Pt, Pd, Ni/Mg0.85Ce0.153+O | 135 | 160 | 490 | 510 | 735 | 737.7 |
Fig. 3b–d and Table 3 illustrate the TPR profile for the catalysts including the promoter Ce2O3. The TPR profiles for Pt, Pd, Ni/Mg0.97Ce0.033+O, Pt, Pd, Ni/Mg0.93Ce0.073+O, and Pt, Pd, Ni/Mg0.85Ce0.153+O catalysts are quite different from the catalyst Pt, Pd, Ni/MgO. The findings show five peaks. The first three peaks of the catalyst Pt, Pd, Ni/Mg0.97Ce0.033+O were recorded at 129 °C, 169 °C, and 481 °C; whilst the peaks of the catalyst Pt, Pd, Ni/Mg0.93Ce0.073+O were recorded at 132 °C, 198 °C, and 495 °C. Meanwhilst, the peaks of the catalyst Pt, Pd, Ni/Mg0.85Ce0.15O were recorded at 135 °C, 160 °C, and 490 °C. This was due to the reduction of PtO, PdO, and NiO on the surface of the catalysts to obtain the elements Pt0, Pd0, and Ni0, respectively. The fourth peak of the catalysts Pt, Pd, Ni/Mg0.97Ce0.033+O, Pt, Pd, Ni/Mg0.93Ce0.073+O, and Pt, Pd, Ni/Mg0.85Ce0.153+O was found where the temperatures were recorded at 502 °C, 508 °C, and 510 °C, respectively. The findings correspond to the reduction of Ce2O3 on the surface. In fact, there was a significant lowering of the surface of Ce2O3 when there was a reduction in the temperature of the catalysts. There are several possible explanations for this phenomenon. Firstly, it could be due to the improved dispersion of Ce2O3 particles during the incorporation of MgO into Ce2O3 and the retardation of sintering.29 Secondly, it could be due to the strong interaction between Ce2O3 and Pt, Pd, and Ni metals; these occur during the overlapping of the PtO, PdO and NiO and Ce2O3 in the reduction of peaks. The fifth peak was recorded at temperatures of 711 °C, 727 °C, and 735 °C. This is attributed to the reduction of bulk Ce2O3 that led to strong interactions between the species of the promoter, Ce2O3, and the support, MgO. It has been revealed that the catalysts show more reducibility with an increase in the loading of the promoter. This finding is in agreement with the results of previous research studies, as compared with the previous study of the cerium reduction results of Rotaru et al.,29 where the reduction of cerium took place at 490 °C and 790 °C. There is a good dispersion of promoters to the support, and it induces a high level of interaction between the support with doping Pt, Pd, and Ni species. Indeed, the significant peak of the TPR profile recorded at temperatures between 684–737 °C proves that Ce2O3 alone can reduce the range in temperatures.30 It is also obvious that the addition of the promoter, Ce2O3, is effective in the reducibility of catalysts with MgO support. This could be attributed to the acidic–basic properties of the support. It has been found that Mg1−xCex3+O with a higher basicity than MgO interacts more with the Ce2O3 promoter. Thus, the reductions in PtO, PdO, and NiO are more obvious. This is due to the redox property of Mg1−xCex3+O.31
The total amount of H2-consumption in the reduction of Pt, Pd, Ni/MgO, Pt, Pd, Ni/Mg0.97Ce0.033+O, Pt, Pd, Ni/Mg0.93Ce0.073+O, and Pt, Pd, Ni/Mg0.85Ce0.153+O was calculated from the total area of the peaks. The calculations for the catalysts were 488.6, 429.1, 941.9, and 737.7 μmol g−1 catalyst, respectively. Based on the TPR-H2 results, the catalyst Pt, Pd, Ni/Mg0.93Ce0.073+O shows that it is the most active site amongst the other catalysts. In other words, it is the best catalyst for dry reforming of methane.
| Sample name | Specific surface area (m2 g−1) | Pore volume (cm3 g−1) | Pore radius (oA) |
|---|---|---|---|
| MgO | 11.1 | 0.21 | 9.9 |
| Pt, Pd, Ni/MgO | 12.4 | 0.21 | 9.7 |
| Pt, Pd, Ni/Mg0.97Ce0.033+O | 12.7 | 0.21 | 13.2 |
| Pt, Pd, Ni/Mg0.93Ce0.073+O | 19.8 | 0.22 | 12.5 |
| Pt, Pd, Ni/Mg0.85Ce0.153+O | 12.9 | 0.21 | 12.4 |
| Spent catalyst | 20.2 | 0.23 | 15.6 |
Table 4 shows the pore radius of the different catalysts. The pore size of the support MgO was recorded at 9.9 Å; whereas, the pore size of the catalyst Pt, Pd, Ni/MgO was recorded at 9.7 Å. The pore radius of the remaining catalysts was inversely proportionate to the increase in the support of the promoter, Ce2O3; whereby, the pore radii of the catalysts: Pt, Pd, Ni/Mg0.97Ce0.033+O, Pt, Pd, Ni/Mg0.93Ce0.073+O and Pt, Pd, Ni/Mg0.85Ce0.153+O were recorded at 13.2 Å, 12.5 Å, and 12.4 Å, respectively.33 This shows that the catalyst Pt, Pd, Ni/Mg0.93Ce0.07O with a high surface area was able to perform better in the dry reforming of the methane reaction as compared to the other catalysts.
3.2. Catalytic performance in biogas reforming
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 (2
CO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), respectively, and the H2
1), respectively, and the H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO ratio of 1.15. However, the conversion of the gases, CH4
CO ratio of 1.15. However, the conversion of the gases, CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 at the ratio of (1
CO2 at the ratio of (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was 84%, 99% and 1.2, respectively. This indicates that the ratio of 1
1) was 84%, 99% and 1.2, respectively. This indicates that the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
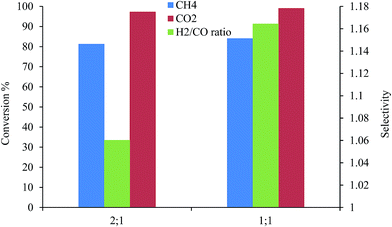
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 provides the best resistance to the deactivation of the catalyst. The reason for this is the carbon formation and high selectivity of H2 and CO (Fig. 6). The other catalysts have also been shown to be similar in this aspect.38
1 provides the best resistance to the deactivation of the catalyst. The reason for this is the carbon formation and high selectivity of H2 and CO (Fig. 6). The other catalysts have also been shown to be similar in this aspect.38
| Sample name | Ni% | Pd% | Pt% | Mg & Ce% |
|---|---|---|---|---|
| Pt, Pd, Ni/MgO | 1.23 | 1.09 | 1.15 | 96.30 |
| Pt, Pd, Ni/Mg0.97Ce0.033+O | 1.18 | 1.11 | 1.19 | 96.17 |
| Pt, Pd, Ni/Mg0.93Ce0.073+O | 1.07 | 1.10 | 1.15 | 96.7 |
| Pt, Pd, Ni/Mg0.85Ce0.153+O | 1.13 | 1.22 | 1.26 | 96.1 |
The Pt and Pd were combined with nickel on the support, MgO–Ce2O3. The experiments were conducted under the following conditions: a temperature of 900 °C and 1 atm with a feed ratio (CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2) of 1
CO2) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
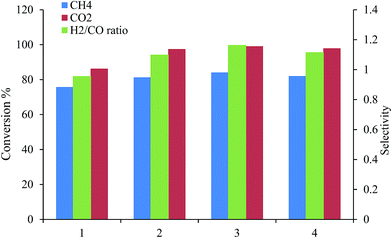
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 7 & Table 5). As for the conversion of methane, the highest conversion recorded was found in the catalyst Pt, Pd, Ni/Mg0.93Ce0.073+O (84%); whilst, the lowest conversion was observed in the catalyst Pt, Pd, Ni/MgO (75%). Another finding is that most of the catalysts tested showed a slight deactivation after 200 h. Overall, the conversion of CO2 was more stable than for methane; and, for the conversion of CH4, the highest conversion was found in the catalyst Pt, Pd, Ni/Mg0.93Ce0.073+O (99%); whilst, the lowest conversion was observed in the catalyst Pt, Pd, Ni/MgO (86%). From the readings, it can be concluded that the best catalyst is Pt, Pd, Ni/Mg0.93Ce0.073+O.
1 (Fig. 7 & Table 5). As for the conversion of methane, the highest conversion recorded was found in the catalyst Pt, Pd, Ni/Mg0.93Ce0.073+O (84%); whilst, the lowest conversion was observed in the catalyst Pt, Pd, Ni/MgO (75%). Another finding is that most of the catalysts tested showed a slight deactivation after 200 h. Overall, the conversion of CO2 was more stable than for methane; and, for the conversion of CH4, the highest conversion was found in the catalyst Pt, Pd, Ni/Mg0.93Ce0.073+O (99%); whilst, the lowest conversion was observed in the catalyst Pt, Pd, Ni/MgO (86%). From the readings, it can be concluded that the best catalyst is Pt, Pd, Ni/Mg0.93Ce0.073+O.
The product ratio of H2/CO over all the tri-metallic magnesia-ceria catalysts (Fig. 7 & Table 5) was recorded at above 1. This indicated that the process of CO2 conversion of the Ni metal was less favourable than that of the tri-metallic catalysts as compared to the other studies.28 Also, the side reactions showed improvement. This is evident from the observed difference between the conversions and the product yields. Table 5 shows that with an increase in the concentration of ceria, there were increases in the conversion rate of CH4 and CO2 as well as the ratio of H2/CO. The best result observed by the catalyst Pt, Pd, Ni/Mg0.93Ce0.073+O was found at the most active site of H2-TPR and from the large surface area indicated from the BET result.
This phenomenon reveals that the incorporation of Ce2O3 into the MgO catalysts can significantly depress the Reverse Water Gas Shift (RWGS) reaction (eqn (11)).
| CO2 + H2 → CO + H2O, ΔH°298K = 41.0 kJ mol−1 | (11) |
The results also indicate that the rate of the CO formation in the dry reforming of methane was dependent on the strong interaction between the promoter, Ce2O3, and the support, MgO solid solution, based on the following findings: the ratio of 0.07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.93 mole in the catalyst, the largest surface area of 19.8 m2 g−1 (Table 4), and the most active site of 941.9 μmol g−1 from the total amount of H2-consumption in the H2-TPR study (Table 3). Hence, the formation of a solid solution was crucial in the generation of the active sites for the CO2 reforming of methane.
0.93 mole in the catalyst, the largest surface area of 19.8 m2 g−1 (Table 4), and the most active site of 941.9 μmol g−1 from the total amount of H2-consumption in the H2-TPR study (Table 3). Hence, the formation of a solid solution was crucial in the generation of the active sites for the CO2 reforming of methane.
This took place because the entire promoter, Ce2O3, was present as a solid solution, which stabilised both oxides. Only the surface layer of Ce2O3 of the solid solution of the catalyst Ce2O3–MgO was reduced during the reduction of hydrogen at 700 °C. Furthermore, the Ce sites generated remained in close contact with the solid solution, which became a hindrance to the Ce sintering.39 Furthermore, the sites that were responsible for the catalytic process can be found in the Pt, Pd, and Ni particles. This is because the Pt, Pd, and Ni particles have a strong interaction with MgO–Ce2O3. When there was an increase in the concentration of Pt, Pd, and Ni in the support, there were no significant changes in the CH4 and CO2 conversion and selectivity. This can be attributed to the formation of nanoparticles as a result of the XRD results (see Debye Sherrer's equation) and TEM results (see Table 2). It is known that the size distribution obtained from TEM images tends to be more realistic and accurate. However, the images have presented some limitations. On the one hand, ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) X-ray diffraction provided a very simple possible estimation of the crystal size from the broadening of the XRD reflections through the use of Scherrer's formula. On the other hand, nanoparticulation of the particles is a preferred choice. The use of the selected nanoparticles as catalysts for this research study not only maximised the surface area but secured more reaction. Also, it ensured a good dispersion of the Pt, Pd, and Ni metals on the surface of the catalysts, as well as strong Lewis basicity with metal oxide support. The increase of the support in Lewis basicity enhanced the ability of the catalyst to chemisorb CO2 in the dry reforming of methane. Also, the adsorbed CO2 reacted with C to form CO (eqn (12)), resulting in the reduction in coke formation.
X-ray diffraction provided a very simple possible estimation of the crystal size from the broadening of the XRD reflections through the use of Scherrer's formula. On the other hand, nanoparticulation of the particles is a preferred choice. The use of the selected nanoparticles as catalysts for this research study not only maximised the surface area but secured more reaction. Also, it ensured a good dispersion of the Pt, Pd, and Ni metals on the surface of the catalysts, as well as strong Lewis basicity with metal oxide support. The increase of the support in Lewis basicity enhanced the ability of the catalyst to chemisorb CO2 in the dry reforming of methane. Also, the adsorbed CO2 reacted with C to form CO (eqn (12)), resulting in the reduction in coke formation.
| CO2 + C → 2CO, ΔH298K° = +172 kJ mol−1 | (12) |
The formation of the Ce2O3–MgO solid solution served as a unique approach in the prevention of carbon deposition. MgO is a strong Lewis base, with a high adsorption of CO2 in reducing or preventing carbon deposition. Furthermore, the XPS results revealed that the reduction of Ce2O3 in the solid solution of Ce2O3–MgO was much more difficult, often leading to the formation of smaller particles of cerium on the surface than that of pure Ce2O3.40,41 The combination of the surface basicity and the particle size of the small metals made-up the ability of the catalyst, MgO-based solid solution, in the prevention of carbon deposition. Although the size distribution obtained from the TEM images tended to be more realistic and accurate, there were some setbacks in the images.
Furthermore, the conversion of CH4 and CO2 was very high due to the particle size involved in the reactive activity. The doping metals, Pt, Pd, and Ni, were prepared based on the Debye Sherrer equation, and supported by the TEM analysis. The metal size had to be as minute as nanoparticles. Thus, it is evident that particle size plays a significant role in the activity of the reaction. An increase in the conversion of the reactants and selectivity (yield) was mainly due to the reduction of the particles in nano-ranged sizes leading to an increase in active sites which was recorded at 941.9 μmol g−1, and in the surface area which was recorded at 19.8 m2 g−1 (refer to Tables 3 and 4).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 (1
CO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) increased as the temperature was raised from 700 °C to 900 °C. This was because the dry reforming of the methane reaction showed a strong endothermic reaction (eqn (3)) and a higher temperature increased the conversion rate. This has been observed in previous studies.42 When the temperature was increased from 700 °C to 900 °C, the CH4 conversion of Pd,Pd,Ni/Mg0.93Ce0.073+O showed an increase from 60% to 84%; whilst the CO2 conversion showed an increase from 38% to 99%. At temperatures above 900 °C, there was no significant increase in the conversions of CH4 and CO2. Fig. 8 shows the H2/CO ratio of the catalyst at various temperatures; when the temperature was <900 °C, the H2/CO ratio of the samples was recorded as <1. This was because the reverse water gas shift reaction (RWGS), (eqn (11)) consumed additional H2 and produced CO; thereby lowering the H2/CO ratio. At a temperature of 900 °C, the H2/CO ratio of Pd,Pd,Ni/Mg0.93Ce0.073+O was recorded at 1.16, indicating a smaller contribution from the RWGS reaction (eqn (11)).15
1) increased as the temperature was raised from 700 °C to 900 °C. This was because the dry reforming of the methane reaction showed a strong endothermic reaction (eqn (3)) and a higher temperature increased the conversion rate. This has been observed in previous studies.42 When the temperature was increased from 700 °C to 900 °C, the CH4 conversion of Pd,Pd,Ni/Mg0.93Ce0.073+O showed an increase from 60% to 84%; whilst the CO2 conversion showed an increase from 38% to 99%. At temperatures above 900 °C, there was no significant increase in the conversions of CH4 and CO2. Fig. 8 shows the H2/CO ratio of the catalyst at various temperatures; when the temperature was <900 °C, the H2/CO ratio of the samples was recorded as <1. This was because the reverse water gas shift reaction (RWGS), (eqn (11)) consumed additional H2 and produced CO; thereby lowering the H2/CO ratio. At a temperature of 900 °C, the H2/CO ratio of Pd,Pd,Ni/Mg0.93Ce0.073+O was recorded at 1.16, indicating a smaller contribution from the RWGS reaction (eqn (11)).15
| CH4 + 2Ni(as) → CH3Ni(as) + HNi(as) |
| CH3Ni(as) + Ni(as) → CH2Ni(as) + HNi(as) |
| CH2Ni(as) + Ni(as) → CHNi(as) + HNi(as) |
| CHNi(as) + Ni(as) → CNi(as) + HNi(as) |
| 2HNi(as) → H2(g) + 2Ni(as) |
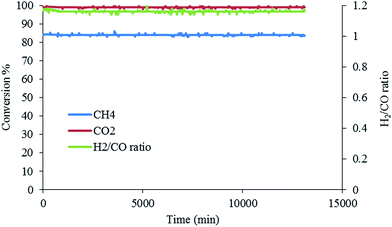 | ||
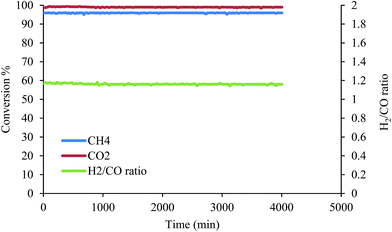
Fig. 9 Stability tests of Pt, Pd, Ni/Mg0.93Ce0.073+O catalysts at 900 °C for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of CH4 1 ratio of CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2, for 200 h. (GHSV = 15 CO2, for 200 h. (GHSV = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ml gcat−1 h−1, atmospheric pressure). 000 ml gcat−1 h−1, atmospheric pressure). | ||
Nakamura et al.44 stated the effects of a promoter in the catalyst on the dry reforming of methane. One of them was by increasing the dispersion of Pt, Pt and Ni, carbon dioxide was activated on the support-promoter in proximity to the metal particle to form a carbonate species. Following that, the CHx species reduced the carbonate to form carbon monoxide (CO).
| CO2(g) → CO2(support) |
| CO2(support) + O2−(support) → CO32−(support) |
| 2H(metal) → 2H(support) |
| CO32−(support) + 2H(support) → HCO2−(support) + OH−(support) |
| CO(support) → CO(g) |
The role of the promoter Ce2O3 in the catalyst was to provide a highly stable platform and a strong resistance to coking that takes place during the conversion of CO2 and CH4 as well as a H2/CO ratio for over 200 h of reaction. The formation of carbon on the catalyst during the dry reforming of the methane reaction was removed by Ce2O3. Also, the CO2 adsorption was enhanced in the presence of Ce2O3 as Ce2O3 can increase the basicity. The next stage after adsorption, was the formation of the carbonate species. It occurred mostly on Ce2O3; whereby, it dissociated to CO2 and then to CO and O. Then O atom is transferred to Ce promoter and finally combine with the C which was deposited on the metal catalyst to produce CO.45 It was evident that there was remarkable decrease of carbon deposition on the catalyst. When the concentration of the Ce2O3 was low, the CO2 conversion showed an increase in the formation of strong ionic oxides, Ce2O2CO3, which resulted in the attraction of CO2 to the surface of the catalyst thereby increasing CH4 conversion. When the concentration of Ce2O3 was high, the conversion of both CH4 and CO2 showed a decrease. This probably took place because of an increase in the electron density of Pt, Pd, and Ni.46 The Ce2O2CO3 species participated directly in DRM by decomposing to produce CO and to provide an oxygen species to react with the carbon deposits on the interface of Pt, Pd, and Ni–Ce2O2CO3; thus, restoring the activity of the Pt, Pd, and Ni sites. Similarly, the Ce2O3 supported catalysts can facilitate the dissociation of adsorbed CO2. In addition to its promotional effect on CO2, the dissociative adsorption of ceria can also improve the dispersion and stabilisation of small metal particles. In fact, ceria is one of the oxides known to exert strong interactions on the supported metallic phase, resulting in significant alterations of the surface properties of both the oxide and metal.47
| CO2(g) → CO(support) + O(promoter) |
| C(metal) + O(promoter) → CO2(g) |
The activity and stability of the tri-metallic catalyst Ni–Pd–Pt was much higher than that of the mono-metallic catalyst Ni, or bi-metallic catalysts Pd–Ni and Pt–Ni. This is consistent with the hypothesis that Pt and Pd help prevent oxidation of Ni due to an increase in its electron density.48 The formation of a Pt–Ni or Pd–Ni bi-metallic cluster increases the reducibility of Ni, resulting in higher and more stable activity for experiments of over 200 h.49
Fig. 10 shows the TGA results with an oxygen stream of post-reaction tri-metallic catalyst Pt, Pd, Ni/Mg0.93Ce0.073+O together with the calculation of weight change at each temperature range illustrated at the thermo-gram. There are altogether three different regions of temperature. The first region is the low temperature range. Here, the spent catalyst experienced an increase in weight. The second region is the mid temperature range. The spent catalyst experienced a decrease in weight in this region. The third region is the high temperature range. The spent catalyst found here experienced an increase in weight. The oxidation of the Ni particles at temperatures above 100 °C caused the increase in weight. Zhu et al.50 reported the increase in weight of the spent catalyst as less than 1%. The compound was found to be stable in the range of the temperatures (150–500) °C; whilst the loss of weight at a temperature of 650 °C was due to the oxidation of deposited carbon. It was found that the carbon deposited on the spent catalyst was layered carbon, with further support in the absence of filamentous carbon from the TEM results of the spent catalysts. The amount of coke deposited on the spent catalyst was calculated to be 2.4 wt%. Finally, all elements on the surface were synthesized by oxygen after the removal of carbon on the surface as CO2. In conclusion, the results showed that at 900 °C, there was little deposition of coke on the surface of the catalyst, and the formation of the coke was related to the dispersion of metal in the catalysts. The size of the smaller crystal metal catalysts makes it less prone to deactivation.
| CH4 + 1.5O2 → CO + 2H2O(v) | (13) |
| C(s) + H2O → CO + H2 | (14) |
| C(s) + O2 → CO2 | (15) |
4. Conclusions
It is inevitable that biogas is an attractive carbon source for the production of clean fuels and chemicals because it is renewable, easily available, and inexpensive. In addition, its processing, storage and usage employs technologies and infrastructure that are developed for natural gas. Meanwhilst, the dry reforming of biogas is a promising technology in the production of syngas. In terms of synthesized carbon, this approach is highly beneficial, as both major constituents of biogas (CH4 and CO2) are incorporated into the final hydrocarbon product. The main catalysts of Ni, Pd, Pt supported on MgO and MgO–Ce2O3 with cubic structures that were synthesized using the co-precipitation method with K2CO3 as the precipitant has yielded good CO2 and CH4 conversion rates of 99% and 80%, respectively, for DRM at 900 °C. They also showed good thermal stability for the first 200 h. The supported catalysts, Pt, Pd, Ni/MgO and Pt, Pd, Ni/MgO–Ce2O3, with cubic structures showed good activity and thermal stability for the types of dry reforming of methane and the partial oxidation of methane reactions. These two steps occur alternately during the reforming process of the methane with the catalysts: Pt, Pd, Ni/MgO and Pt, Pd, Ni/MgO–Ce2O3. This occurrence ensures the deposition of fixed amounts of carbon on the surfaces of the catalysts, which does not affect the activity or thermal stability of the catalysts. These supported catalysts, Pt, Pd, and Ni, with cubic structures have been proven to have great potential for use in fuel processing.Acknowledgements
The authors are thankful of NanoMite Grant (Vot. No: 5526308) for providing the funding to conduct this study. One of the authors also grateful to Basra University, Iraq for giving the financial assistance during his PhD study.References
- K. Sutthiumporn, T. Maneerung, Y. Kathiraser and S. Kawi, Int. J. Hydrogen Energy, 2012, 37, 11195–11207 CrossRef CAS.
- M. R. Rahimpour, Z. A. Aboosadi and A. H. Jahanmiri, Appl. Energy, 2011, 88, 2691–2701 CrossRef.
- W. H. Chen, B. J. Lin, H. M. Lee and M. H. Huang, Appl. Energy, 2012, 98, 92–101 CrossRef CAS.
- M. Sarkari, F. Fazlollahi, H. Ajamein, H. Atashi, W. C. Hecker and L. L. Baxter, Fuel Process. Technol., 2014, 127, 163–170 CrossRef CAS.
- E. R. Monazam, R. W. Breault and R. Siriwardane, Chem. Eng. J., 2014, 242, 204–210 CrossRef CAS.
- A. Hasanbeigi, M. Arens and L. Price, Renewable Sustainable Energy Rev., 2014, 33, 645–658 CrossRef CAS.
- Catal. Sci. Technol, ed. J. R. Rostrup-Nielsen, J. R. Anderson and M. Boudart, Springer, New York, 1984, vol. 5 Search PubMed.
- E. Ruckenstein and Y. H. Hu, Appl. Catal., A, 1995, 133, 149–161 CrossRef CAS.
- D. Dissanayake, M. P. Rosynek and L. H. Lunsford, J. Phys. Chem., 1993, 97, 3644–3646 CrossRef CAS.
- A. T. Ashcroft, A. K. Cheetham, M. H. Green and P. F. Vernon, Nature, 1991, 352, 225–231 CrossRef CAS.
- Q. Chen, J. Zhang, Q. Jin, B. Pan, W. Kong, T. Zhao and Y. Sun, Catal. Today, 2013, 215, 251–259 CrossRef CAS.
- J. Ashok and S. Kawi, Int. J. Hydrogen Energy, 2013, 38, 13938–13949 CrossRef CAS.
- Y. Liu, Z. He, L. Zhou, Z. Hou and W. Eli, Catal. Commun., 2013, 42, 40–44 CrossRef CAS.
- Q. Chen, J. Zhang, Q. Jin, B. Pan, W. Kong, T. Zhao and Y. Sun, Catal. Today, 2013, 215, 251–259 CrossRef CAS.
- J. Kehres, J. G. Jakobsen, J. W. Andreasen, J. B. Wagner, H. Liu, A. Molenbroek and T. Vegge, J. Phys. Chem. C, 2012, 116, 21407–21415 CAS.
- M. Garcia-Dieguez, I. S. Pieta, M. C. Herrera, M. A. Larrubia and L. J. Alemany, Catal. Today, 2011, 172, 136–142 CrossRef CAS.
- F. Menegazzo, M. Signoretto, P. Canton and N. Pernicone, Appl. Catal., A, 2012, 439, 80–87 CrossRef.
- E. Ruckenstein and Y. H. Hu, Chem. Innovation, 2000, 30, 39–43 CAS.
- M. Yu, K. Zhu, Z. Liu, H. Xiao, W. Deng and X. Zhou, Appl. Catal., B, 2014, 148, 177–190 CrossRef.
- K. Tomishige, Catal. Today, 2004, 89, 405–418 CrossRef CAS.
- F. W. Aldbea, N. Ibrahim, M. H. Abdullah and R. E. Shaiboub, J. Sol-Gel Sci. Technol., 2012, 62, 483–489 CrossRef CAS.
- P. Grange, Catal. Rev.: Sci. Eng., 1980, 21, 135–181 CAS.
- H. Abimanyu, C. S. Kim, B. S. Ahn and K. S. Yoo, Catal. Lett., 2007, 118, 30–35 CrossRef CAS.
- X. Chen, J. Jiang, S. Tian and K. Li, Catal. Sci. Technol., 2015, 5, 860–868 CAS.
- M. Zhiijian, L. Ying, F. Maohong and Z. Ling, J. Chem. Eng., 2015, 259, 293–302 CrossRef.
- C. Hidalgo, S. Jalila, M. Alberto, M. Jose and S. Said, J. Colloid Interface Sci., 2012, 382, 67–73 CrossRef PubMed.
- E. G. Mahoney, J. M. Pusel, S. M. Stagg-Williams and S. Faraji, J. CO2 Util., 2014, 6, 40–44 CrossRef CAS.
- Z. Bao, Y. Lu, J. Han, Y. Li and F. Yu, Appl. Catal., A, 2015, 491, 116–126 CrossRef CAS.
- C. G. Rotaru, G. Postole, M. Florea, F. Matei-Rutkovska, V. I. Pârvulescu and P. Gelin, Appl. Catal., A, 2015, 494, 29–34 CrossRef CAS.
- S. Tada, T. Shimizu, H. Kameyama and T. Haneda, Int. J. Hydrogen Energy, 2012, 37, 5527–5531 CrossRef CAS.
- V. M. Gonzalez-Delacruz, F. Ternero, R. Peren, A. Caballero and J. P. Holgado, Appl. Catal., A, 2010, 384, 1–9 CrossRef CAS.
- K. Y. Koo, H. S. Roh, Y. T. Seo, D. J. Seo, W. L. Yoon and S. B. Park, Int. J. Hydrogen Energy, 2008, 33, 2036–2043 CrossRef CAS.
- Z. Mei, Y. Li, M. Fan, L. Zhao and J. Zhao, Chem. Eng. J., 2015, 259, 293–302 CrossRef CAS.
- A. Djaidja, S. Libs, A. Kiennemann and A. Barama, Catal. Today, 2006, 113, 194–200 CrossRef CAS.
- H. W. Kim, K. M. Kang and H. Kwak, Int. J. Hydrogen Energy, 2009, 34, 3351–3359 CrossRef CAS.
- L. Chen, Q. Zhu, Z. Hao, T. Zhang and Z. Xie, Int. J. Hydrogen Energy, 2010, 35, 8494–8502 CrossRef CAS.
- Z. Mojovic, S. Mentus and Z. Tesic, Mater. Sci. Forum, 2004, 453, 257–262 CrossRef.
- A. M. Gadalla and M. E. Sommer, Chem. Eng. Sci., 1989, 44, 2825–2829 CrossRef CAS.
- A. Zecchina, G. Spoto, S. Coluccia and E. Guglielminotti, J. Chem. Soc., Faraday Trans., 1984, 80, 1891–1901 RSC.
- Y. H. Hu and E. Ruckenstein, Acc. Chem. Res., 2003, 36, 791–797 CrossRef CAS PubMed.
- S. Appari, V. M. Janardhanan, R. Bauri, S. Jayanti and O. Deutschmann, Appl. Catal., A, 2014, 471, 118–125 CrossRef CAS.
- P. Djinović, G. Osojnik, B. Erjavec and A. Pintar, Appl. Catal., B, 2012, 125, 259–270 CrossRef.
- A. Topalidis, D. E. Petrakis, A. Ladavos, L. Loakatzikou and P. J. Pomonis, Catal. Today, 2007, 127, 238–245 CrossRef CAS.
- K. Nakagawa, M. Kikuchi, M. Nishitani-Gamo, H. Oda, H. Gamo, K. Ogawa and T. Ando, Energy Fuels, 2008, 22, 3566–3570 CrossRef CAS.
- T. Osaki and T. Mori, J. Catal., 2001, 204, 89–97 CrossRef CAS.
- M. M. Barroso-Quiroga and A. E. Castro-Luna, Int. J. Hydrogen Energy, 2010, 35, 6052–6056 CrossRef CAS.
- F. Giordano, A. Trovarelli, C. Leitenburg and M. Giona, Catalysis, 2000, 193, 273–282 CrossRef CAS.
- B. Steinhauer, M. R. Kasireddy, J. Radnik and A. Martin, Appl. Catal., A, 2009, 366, 333–341 CrossRef CAS.
- S. R. Miguel, I. M. J. Vilella, S. P. Maina, D. S. Jose-Alonso, M. C. Roman-Martinez and M. J. Illan-Gomez, Appl. Catal., A, 2012, 435, 10–18 CrossRef.
- J. Zhu, X. Peng, L. Yao, J. Shen, D. Tong and C. Hu, Int. J. Hydrogen Energy, 2011, 36, 7094–7104 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |