Water soluble EDTA-linked chitosan as a zwitterionic flocculant for pH sensitive removal of Cu(ii) ion
Abstract
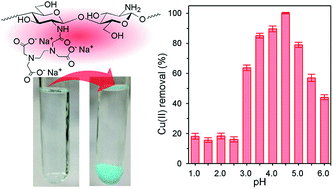
A linear and non-crosslinked polymer, EDTA-linked chitosan (ED-ch), was synthesized by N-acylation of chitosan with EDTA (ethylenediaminetetraacetic acid) monoanhydride under acidic to slightly basic aqueous conditions. The degree of substitution (D.S.) of EDTA residues in the product was changeable depending on the stoichiometry of the acylation reagent, and the structure was confirmed by elemental analysis, FT-IR, 1H and 13C NMR spectroscopy. The newly introduced functional group in ED-ch provided properties such as being a strong chelating reagent and an amphoteric polyelectrolyte. It was found that ED-ch had good water solubility in both acidic and basic regions and precipitated in a narrow pH region. Applicability of ED-ch to the removal of heavy metal ions by flocculation was proven by a model experiment using 10 ppm Cu(II) solution. A simple procedure, mixing ED-ch with D.S. 41% and 70%, and subsequent pH adjustment (4.5 and 2.5) and centrifugal separation of the flocs formed, was shown to remove Cu(II) almost completely from aqueous solution. The residual Cu(II) concentration after these flocculation/separation process with ED-ch reached 0.03 ppm.

 Please wait while we load your content...
Please wait while we load your content...