Interactions governing the entrapment of anticancer drugs by low-molecular-weight hydrogelator for drug delivery applications
Abstract
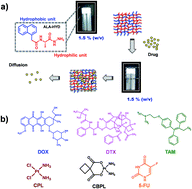
We present the effect of size, charge, and hydrophobicity of anticancer drugs on their drug encapsulation efficacy in an L-alanine-based small-molecule hydrogelator. Entrapment of various anticancer drugs in a hydrogel was depicted and correlated towards interactions between gelator and drug molecules. Hydrogel showed the highest entrapment for 5-fluorouracil, which was as high as ∼1.2 mg mL−1 in 1.5% (w/v) hydrogel; however, with small polar anticancer drugs such as cisplatin and carboplatin, poor encapsulation was observed. Hydrogel was also able to entrap and retain hydrophobic drugs, such as docetaxel and tamoxifen, with a high drug-loading efficiency. The hydrogel-entrapped drugs were then characterized by rheology and SEM studies to understand the effect of the drugs on hydrogel assembly. Drug release and anticancer activity studies showed slow and sustained release of drugs from hydrogels, making them suitable for exploring with regard to future cancer therapeutic applications.

 Please wait while we load your content...
Please wait while we load your content...