A facile one-step synthesis of fluorescent silicon quantum dots and their application for detecting Cu2+†
Bo Liao*ac,
Wu Wanga,
Xiaoting Denga,
Benqiao He*b,
Wennan Zenga,
Zilong Tangc and
Qingquan Liua
aSchool of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan, 411201, China. E-mail: lb@hnust.edu.cn; Tel: +86-731-58290045
bState Key Laboratory of Separation Membranes and Membrane Processes, Tianjin Polytechnic University, Tianjin, 300387, China. E-mail: hebenqiao@tjpu.edu.cn; Tel: +86-22-83955055
cKey Laboratory of Theoretical Organic Chemistry and Functional Molecule, Ministry of Education, Hunan University of Science and Technology, Xiangtan, 411201, China
First published on 20th January 2016
Abstract
Silicon quantum dots (Si QDs) were synthesized by a facile one-step synthesis that involved etching silicon powder through a hydrothermal method. The Si QDs, without any surface modification, maintained good water dispersibility, strong photoluminescence and high pH stability, and can be used as a sensor to detect Cu2+.
Silicon quantum dots (Si QDs), a zero-dimensional silicon nano-material, have attracted increasing interest for their potential applications in photonic circuits,1,2 solar energy,3 and nonlinear optics,4 owing to their excellent photoluminescence properties; they have attracted especial interest in the biological and biomedical fields5 because of their favorable biocompatibility and low toxicity.6,7 In the past several years, many methods of synthesizing Si QDs have been reported, including chemical etching of Si wafers,8 electrochemical etching of Si wafers,9 pulsed laser ablation of Si wafers,10 low-pressure plasma synthesis,11 atmospheric-pressure microdischarge synthesis,12 thermal processing of silesquioxanes,13 microwave synthesis14,15 and solution phase methods.16 Among these reported methods, microwave synthesis should be a simple option. However, in the microwave synthesis methods that have been reported, silicon nanowires are used as precursors and must be firstly prepared by chemical etching Si wafers before preparing the Si QDs,13 or the prepared Si QDs must be further passivated for water solubility.14 Chemical etching to prepare fluorescent Si QDs is a dangerous method because hazardous chemicals such as HF must be used to etch the Si wafers. Meanwhile, pulsed laser ablation and low-pressure plasma synthesis require expensive instruments, and further processing is required to obtain fluorescent Si QDs. Therefore, these reported methods require complicated procedures, expensive precursors, harsh synthetic conditions or surface modifications that hinder their development. It is necessary to develop a simple and convenient method, which can overcome those disadvantages and be expanded to synthesize Si QDs for broad applications.
Herein, we reported an incredibly simple, convenient, economic, and lab accessible one-step synthesis method for the synthesis of Si QDs simply by employing hydrothermal etching of silicon powder. The as-prepared Si QDs have an average diameter of 2.3 nm and show strong photoluminescence, high pH stability and good aqueous dispersibility without any surface modification or further processing. Moreover, we found that the fluorescence of the as-prepared Si QDs could be quenched by metal Cu2+ ions; in the presence of phytic acid, they could be used as a sensor to detect Cu2+. The as-prepared Si QDs have potential applications in the fields of optoelectronics, biomedicine, and biology as sensors for Cu2+ ion monitoring.
Fig. 1 displays transmission electron microscopy (TEM) images of Si QDs prepared by etching Si powder for 2 h at 200 °C, as well as the pristine Si powder. The Si QDs are spherical particles that appear to have low dispersity and a diameter of about 2–3 nm, whereas the pristine Si powder is irregularly shaped and far greater in size than the Si QDs. The size distribution given in Fig. S2† (calculated by measuring more than 300 particles) shows that the Si QDs have an average size of 2.3 nm. Meanwhile, we found that although we etched the Si powder for different lengths of time, the prepared Si QDs have almost the same size (Fig. S3†). This indicates that the size of the Si QDs is not influenced by etching time.
 | ||
| Fig. 1 TEM images of the Si QDs prepared by etching for 2 h at 200 °C (left) and the pristine Si powder (right). | ||
The UV-visible absorption spectrum of the Si QDs dispersed in water is given in Fig. S4.† It shows that one absorption peak is centered at a wavelength of about 320 nm, while the absorption range is located from 290 to 410 nm. Fig. 2 shows the photoluminescence spectra of the Si QDs prepared by etching Si powder for 2 h when excited at different wavelengths. It is found that the emission bands of the Si QDs locate in the range of 420–600 nm when excited in the range of 260–500 nm. The fluorescence emission of the Si QDs is dependent upon the excitation wavelength in the range of 300–480 nm, which is related to their size-dependent luminescence properties, due to the size distribution of the Si QDs.15 The Si QDs prepared with different etching times have almost the same fluorescence emission properties, as given in Fig. S5;† this indicates that the fluorescence emission properties were not influenced by etching time, which is consistent with the TEM results. The quantum yield of the Si QDs was estimated to be 12% when excited at 340 nm (based on quinine sulfate as a reference at room temperature) and the mean lifetime of the Si QDs was 7.432 ns (λem = 460 nm, λex = 340 nm). Furthermore, the solid powder of the Si QDs can also fluoresce with appropriate excitation wavelengths (Fig. S6†); however, the range of the emission wavelengths became narrow. To exclude the probability that the Si QDs were generated during the grinding process, we also investigated the pristine Si powder; the pristine Si powder did not fluoresce when dispersed in water or in the solid state (Fig. S8†), while at the same time, the size of the pristine Si is far larger than that of the Si QDs (Fig. S3 and S4†). These results confirmed that the fluorescent Si QDs could be indeed synthesized by employing this facile method. We believe that water can react with Si and produce silicic acid due to defects caused by grinding single crystals of Si or from the crystals themselves, at high temperature; that is to say, water is an oxidizing agent, which can etch the single Si crystals into fluorescent Si QDs 2 to 3 nm in size under these hydrothermal conditions.
 | ||
| Fig. 2 The photoluminescence spectra of the Si QDs dispersed in water when excited with different excitation wavelengths. | ||
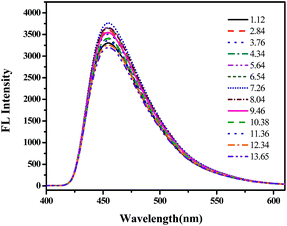
The photoluminescence spectra of the Si QDs dispersed in water at different pH values are given in Fig. 3. Though spanning a wide pH range (1.12–13.65) of acidic-to-basic environments, the peaks of the emission wavelength always appear at 460 nm when excited at 340 nm; the intensity of photoluminescence at 460 nm decreases by only 15%. This shows that the fluorescence emission of the Si QDs is insensitive to variations in pH value. The results show that the Si QDs have robust pH stability and possess stable fluorescence under a wide range of pH values. The robust pH stability may be attributed to the surface-covered hydroxyl groups on the as-prepared Si QDs (as given by the FTIR results in Fig. S7†), which may provide a protective shell for the as-prepared Si QDs and enable them to maintain pH stability.
Fig. 4 shows the relative emission intensity at 460 nm of the Si QDs dispersed in water at pH 7 in the presence of different metal cations (the concentration of the metal cations is 10−4 M). It was found that in the presence of 10−4 M phytic acid, the fluorescence of the Si QDs could be only quenched by Cu2+. However, without phytic acid, most of the metal ions had almost no influence on the fluorescence emission intensity of the as-prepared Si QDs except for Cu2+, Co2+ and Hg2+ (Fig. S8†). These results show that in the presence of phytic acid, the Si QDs display excellent selectivity toward Cu2+ ions, which indicates that the as-prepared Si QDs could be used as a sensor for detecting Cu2+.
On the basis of the above results, we evaluated the sensitivity and linearity of the Si QDs and Cu2+ system by varying the Cu2+ concentration in the presence of 10−4 M phytic acid. As shown in Fig. 5a, the intensity of the fluorescence emission was sensitive and decreased proportionately with increasing concentration of Cu2+. Furthermore, we obtained the linear relationship between the fluorescence intensity at 460 nm and the Cu2+ concentration, as given in Fig. 5b. The linear relation (the range was 0.5–10 M) with an R2 of 0.9986 could be described by the equation (F0 − F)/F0 = KSV[Q], where F and F0 denote the fluorescence intensity at 460 nm with and without Cu2+ respectively, and [Q] denotes Cu2+ concentration. Under the current experimental conditions, the lowest Cu2+ concentration that could be detected was 0.5 μM, which meets the limit of detection requirement for drinking water. Therefore, this system provides a method to detect Cu2+ with high sensitivity and efficiency, and the results indicate that the as-prepared Si QDs can be used as a sensor for detecting Cu2+ in the environment and in the life sciences.
In conclusion, fluorescent Si QDs can be easily synthesized through a facile one-step method of hydrothermal etching silicon powder; the Si QDs can fluoresce blue-green, and possess good aqueous dispersibility and robust pH stability. Meanwhile, the fluorescence emission of the Si QDs is sensitive to Cu2+ in the presence of phytic acid. These Si QDs are promising for applications in many fields, such as optoelectronics, biomedicine, biology and sensors.
Acknowledgements
The authors gratefully acknowledge financial support from the Natural Science Foundation of China (Project No. 21174104 and 51373051), Hunan Provincial Natural Science Foundation of China (13JJ3087), Scientific Research Fund of Hunan Provincial Education Department (No. 15B081) and State Key Laboratory of Separation Membranes and Membrane Processes (Tianjin Polytechnic University) (No. M2-201507).Notes and references
- O. Bisi, S. Ossicini and L. Pavesi, Surf. Sci. Rep., 2000, 38, 1 CrossRef CAS.
- A. Politi, M. J. Cryan, J. G. Rarity, S. Yu and J. L. O'Brien, Science, 2008, 320, 646 CrossRef CAS PubMed.
- X. Pi, L. Zhang and D. Yang, J. Phys. Chem. C, 2012, 116, 21240 CAS.
- G. S. He, Q. Zheng, K. T. Yong, F. Erogbogbo, M. T. Swihart and P. N. Prasad, Nano Lett., 2008, 8, 2688 CrossRef CAS PubMed.
- W. W. Yu, E. Chang, R. Drezek and V. L. Colvin, Biochem. Biophys. Res. Commun., 2006, 348, 781 CrossRef CAS PubMed.
- F. Erogbogbo, K. T. Yong, I. Roy, G. Xu, P. N. Prasad and M. T. Swihart, ACS Nano, 2008, 2, 873 CrossRef CAS PubMed.
- J. H. Warner, A. Hoshino, K. Yamamoto and R. D. Tilley, Angew. Chem., 2005, 117, 4626 CrossRef.
- J. Hwang, Y. Jeong, K. H. Lee, Y. Seo, J. Kim, J. W. Hong, E. Kamaloo, T. A. Camesano and J. Choi, Ind. Eng. Chem. Res., 2015, 54, 5982 CrossRef CAS.
- Y. He, Y. Y. Su, X. B. Yang, Z. H. Kang, T. T. Xu, R. Q. Zhang, C. H. Fan and S. T. Lee, J. Am. Chem. Soc., 2009, 131, 4434 CrossRef CAS PubMed.
- S. Yang, W. Cai, H. Zhang, X. Xu and H. Zeng, J. Phys. Chem. C, 2009, 113, 19091 CAS.
- A. Gupta, M. T. Swihart and H. Wiggers, Adv. Funct. Mater., 2009, 19, 696 CrossRef CAS.
- R. M. Sankaran, D. Holunga, R. C. Flagan and K. P. Giapis, Nano Lett., 2005, 5, 537 CrossRef CAS PubMed.
- C. M. Hessel, E. J. Henderson and J. G. C. Veinot, Chem. Mater., 2006, 18, 6139 CrossRef CAS.
- Y. He, Y. Zhong, F. Peng, X. Wei, Y. Su, Y. Lu, S. Su, W. Gu, L. Liao and S. T. Lee, J. Am. Chem. Soc., 2011, 133, 14192 CrossRef CAS PubMed.
- T. M. Atkins, A. Thibert, D. S. Larsen, S. Dey, N. D. Browning and S. M. Kauzlarich, J. Am. Chem. Soc., 2011, 133, 20664 CrossRef CAS PubMed.
- J. Zou, R. K. Baldwin, K. A. Pettigrew and S. M. Kauzlarich, Nano Lett., 2004, 4, 1181 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, measurements and synthesis and other physical properties data. See DOI: 10.1039/c5ra25563e |
| This journal is © The Royal Society of Chemistry 2016 |