Rational design of photo-responsive supramolecular nanostructures based on an azobenzene-derived surfactant-encapsulated polyoxometalate complex†
Yongxian Guoa,
Yanjun Gonga,
Zhidan Yub,
Yan'an Gaoc and
Li Yu*a
aKey Laboratory of Colloid and Interface Chemistry, Shandong University, Ministry of Education, Jinan 250100, P. R. China
bSchool of Life Science, Shandong University, Jinan 250100, P. R. China
cChina Ionic Liquid Laboratory, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, P. R. China
First published on 26th January 2016
Abstract
Using an ionic self-assembly (ISA) approach, photo-responsive surfactant-encapsulated polyoxometalate complexes (SECs) were fabricated in water from an original Keggin-type polyoxometalate (POM) and a cationic surfactant containing an azobenzene group, viz. phosphotungstic acid (H3[PW12O40]) and 4-ethyl-4′-(trimethylaminohexyloxy) azobenzene bromide (ETAB). The driving forces and self-assembly mechanism of the ETAB–POM supramolecular hybrids were investigated by NMR, Fourier transform infrared (FTIR), UV/vis and small angle X-ray scattering (SAXS) characterization methods. Of particular interest is the complex solution which shows an obvious variation upon UV light irradiation. On a macro-scale, its turbidity increases obviously, from a clear solution before UV irradiation to a turbid state. The microcosmic structures of the complex change from coral-like structures to dispersive nanospheres. These phenomena can be ascribed to the transformation of ETAB from trans- to cis-isomers after exposure to UV light. Beyond that, a cyclic voltammetric (CV) method was employed to observe the electrochemical properties of SECs. The results obtained in this work will shed light of the SECs' applications in phase separation, heterogeneous catalysis reactions, the detection of environmental pollutants, etc.
Introduction
Ionic self-assembly (ISA) has attracted much attention as a crucial strategy to fabricate supramolecular materials. Compared with other time-consuming organic synthesis strategies to prepare different structures with covalent bonds, ISA uses the electrostatic interaction as the main driving force to establish multifarious building blocks. The building blocks further assemble into various architectures with secondary interactions, which include hydrophobic interactions, π–π stacking interactions, hydrogen bonding and charge–transfer interactions.1 Meanwhile, this flexible strategy can introduce some functional groups (e.g. stimuli-responsive groups, unsaturated bonds, chiral groups and so on) into the supramolecules. Therefore, the various fabricated supramolecular architectures possess versatile potential applications.2,3As a kind of newly functional surfactant, stimuli responsive (e.g. photo-, redox-, pH-, CO2-, magneto-, or enzyme-responsive) surfactants have attracted great scientific research interest.4 Thereinto, photo-responsive surfactants containing azobenzenes,5,6 stilbenes7 and spiropyrans groups,8 have got more attention since light is considered as a preeminent trigger (easy to obtain, tune the wavelength and density of light; without environmental damage). Azobenzene derivatives have the specialty of reversible photoinduced isomerization in a certain wavelength of ultraviolet light irradiation, and their dipole moment and polarity can also be changed due to the change of symmetry.9 The structural change can be easily obtained by UV/vis spectroscopy. The intensity of characteristic peak at 365 nm corresponding to π–π* electron transition increases, while that of the band at 420 nm decreases which is ascribed to the n–π* electron transition.5 Therefore, azobenzene derivatives have been employed to fabricate supramolecules via multiple interactions, e.g. electrostatic interaction and guest–host interaction,10,11 etc.
Polyoxometalates (POMs), as a type of nanoscale transition metal oxide cluster, have caused considerable attention owing to their electrochromism,12–14 photochromism,12,15 magnetism,16 and catalysis9,17,18 properties. And POMs, as a negatively charged building units, can be incorporated into appropriate counterions to yield functional materials. The morphologies of the formed materials could be tuned by many regulatory factors, such as the polarity of the solvents, the structures of the building blocks and so on. Wang et al. obtained a dimethyldioctadecylammonium bromide (DODA)-encapsulated complex with variant morphologies (rose-, snow-flower- and ice-ball-like architectures) by tuning the polarity of solvents.19 Cronin and Liu et al. synthesized an inorganic–organic–inorganic polyoxometalates, and assembled into vesicles in acetone/water mixed solution.20 Furthermore, some groups fused responsive groups and POMs as a building block, and the morphological change was realized. For example, Wu and his coworkers synthesized a stimuli-responsive azobenzene grafted Anderson-type cluster, and observed that the SECs' morphology changed from fiber-like to spherical structure upon UV light irradiation.21 Yet, the acquisition of the building blocks needs a time-consuming process because the interaction between the cluster and organic matrices is covalent bond. So far, it has been scarcely reported to build stimuli-responsive surfactant-encapsulated Keggin-type POMs via weak noncovalent interactions.
In the present work, we use ISA approach to prepare SECs with a stimuli-responsive cationic surfactant, 4-ethyl-4′-(trimethylaminohexyloxy) azobenzene bromide (ETAB), and an original Keggin-type polyoxometalate (POM), phosphotungstic acid (H3[PW12O40]) (Scheme 1). The complex solution displays obvious variations before and after UV light irradiation. In the aspect of macroscopic phenomenon, the clear solution before UV irradiation changed into turbid state after UV light irradiation. And the as-prepared coral-like materials transform to dispersive nanospheres, observed by transmission electron microscopy (TEM). The driving force and formation mechanism of ETAB–POM complex were studied by 1H NMR, FTIR, UV/vis and SAXS techniques. Furthermore, the fabricated complex also shows an excellent redox property, certified by cyclic voltammetric (CV) method.
Results and discussion
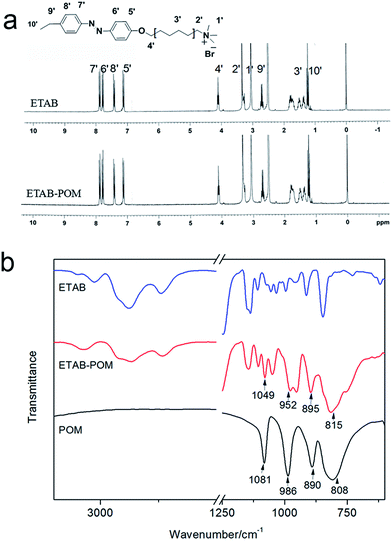
The ETAB–POM nanostructures were fabricated with an ISA strategy by one-step synthesis method: mixing the ETAB and POM solution in equal concentration under stirring. The ETAB–POM mixed solution presented lighter yellow color compared with pure ETAB solution, and showed the typical Tyndall effect (Fig. S1†). It is observed that the coral-like supramolecular materials (Fig. 1a) are composed of many orderly cross-linking individual particles (∼50 nm) (Fig. 1b), as confirmed by TEM studies. To study the formation mechanism and driving forces of the coral-like structures, some characteristic methods were used as follows.To obtain the detail information of hydrogen protons, 1H NMR spectra were measured. As can be seen from Fig. 2a, for the SECs, both the characteristic chemical shifts of azobenzene (δ = 7.1–7.8, 8H, peaks of 5′, 6′, 7′, 8′) and alkyl chain protons of ETAB appear, suggesting that ETAB molecules were not damaged during the process of self-assembly. Meanwhile, the proton peaks of methyl (1′ peak in Fig. 2a) and methylene groups (2′ peak in Fig. 2a) shifted 0.028 and 0.210 ppm, respectively. This can be ascribed to the electrostatic interaction between [ETA]+ ion and negatively charged POM group ([PW12O40]3−). The electronegativity of POM attracts the electron clouds of N–CH3 and N–CH2 groups, leading to a decrease in the electron density. Then it makes the chemical shifts of the protons move slightly to the low field.22
To measure the arrangements of hydrocarbon chains and structural changes of POM in the SECs, FTIR spectra of ETAB, ETAB–POM, and POM are shown in Fig. 2b. From the spectrum of ETAB–POM, the vibration bands appearing at 2938, 2869, and 1469 cm−1 are due to methylene asymmetrical stretching mode νas(CH2), methylene and methyl symmetrical stretching modes, νs(CH2) and νs(CH3), respectively. Based on the literature,23 νas(CH2) (∼2918 cm−1) and νs(CH2) (∼2848 cm−1) are assigned to highly ordered orientation of alkyl chains. Here the positions of these two bands shift to the higher wavenumber, which shows that the ordering of the hydrocarbon chains for ETAB–POM complex reduces. Meanwhile, the bands at 1596 and 1497 cm−1 can be ascribed to the scissoring vibration of C–N on the benzyl group. Furthermore, for POM the bands of 1081, 986, 890, and 808 cm−1 are attributed to ν(P–O), ν(W = Ot), ν(W–Ob–W) and ν(W–Oc–W), respectively.24 The four bands of ETAB–POM at 1049, 952, 895, and 815 cm−1 suggest that the structure of POM is still maintained. The slight shifts are due to the electrostatic interaction between [PW12O40]3− and the cationic head group of ETAB, which was also identified by 1H NMR (Fig. 2a).
As we know, the azobenzene chromophore is a conjugated group with two characteristic peaks. Fig. 1d shows the UV/vis spectra of ETAB solution and ETAB–POM complex solution. The absorption bands around 350 and 430 nm are ascribed to π–π* and n–π* transitions, respectively. The strong adsorption at 351 nm for ETAB has a blue shift to 340 nm in the UV/vis spectrum of ETAB–POM complex, which may be caused by the π–π stacking interaction of the conjugated group in SECs.25
To obtain the information on the molecular arrangement of ETAB–POM hybrid materials, SAXS diffractogram was measured. As shown in Fig. S2,† there are two broad peaks (q = 1.32, 5.41 nm−1), implying the less ordered orientation of lamellar structure. And based on the spacing distance calculated by the equation d = 2π/q, we can obtain d = 4.74 nm. Density functional theory (DFT) calculations via the Gaussian 09 package with the hybrid B3LYP functional and the 6-31G(d, p) basis26 were also performed to evaluate the length of single ETAB molecule. Fig. 3 shows the optimized geometries of trans- and cis-ETAB. The sizes of the isomerizations are about 22.3 Å × 2.5 Å × 2.4 Å and 15.9 Å × 9.0 Å × 6.02 Å, respectively. Based on the size of ETAB molecule and the diameter of single Keggin-type POM molecule (∼1.0 nm), length of the supramolecule formed by trans-ETAB and POM was calculated to be 5.46 nm, which is higher than the spacing distance of ETAB–POM hybrids measured by SAXS, 4.74 nm, but less than twice its value. This indicates an overlap among the hydrophobic chains during the formation process of the supramolecular units (Scheme 1), which also provides a proof of the existence of π–π stacking interaction.
Based on the above experimental results, the driving forces and possible formation mechanism of coral-like ETAB–POM SECs are proposed as follows (Scheme 1): (i) supramolecular units form due to the electrostatic interaction between ETAB and POM molecules. (ii) Nanoparticles self-assemblied by supramolecular units via the π–π stacking interaction, hydrophobic interaction and van der Waals force. (iii) Coral-like structures are fabricated owing to the “hydrophobic bonds”27 between the hydrophobic chains on the surface of the neighboring nanoparticles.
UV-induced morphology change
Through ISA strategy, ETAB with photoisomerization behavior was introduced into the POM-based supramolecular structures. We wonder if the SECs still retain the photo-responsive property. It is observed that the appearance of ETAB–POM aqueous solution changes obviously after irradiation by UV light at 365 nm. There is an evident change in the turbidity. Clear solution before UV irradiation turns into turbid state, accompanied by a color change from faint yellow to dark yellow (Fig. S1†). TEM images demonstrate that the coral-like structures disappeared after exposure to UV light irradiation, and monodispersed nanospheres with a diameter of about 50 nm formed (Fig. 1c). Fig. 1d depicts the UV/vis absorption spectra of ETAB–POM complex solution after different UV irradiation time. Apparently, the curve after UV irradiation for 20 min coincides with that of 30 min, suggesting that the transformation of trans-ETAB–POM comes to extremity after 20 min. The characteristic peak around 340 nm ascribed to π–π* transition remarkably decreases and the absorption at 430 nm assigned to n–π* transition increases slightly with the extension of UV irradiation time. These results can be attributed to the photoisomerization behavior of ETAB molecule in SECs. On one hand, thanks to the discrepancy in the chemical structure of trans- and cis-isomers, this can result in the looser stacking of ETAB molecules after UV light irradiation. On the other hand, as reported, cis-ETAB is less hydrophobic than trans-configuration.28 So the hydrophobic interactions among the hydrophobic chains in the supramolecular units or on the surface of the nanoparticles are weakened. Based on the two reasons as above, it can be concluded that the photoisomerization of ETAB induces the morphology change of the SECs. Scheme 2 shows the schematic drawings of the packing models of ETAB upon UV light irradiation. Unfortunately, when the complex solution was again exposed to visible light, the nanospheres changed into precipitates, instead of coral-like structures. This indicates the irreversible process from nanospheres to the coral-like structures.Electrochemical property of SECs
As reported,29 POMs can undergo multi-electron reduction with small structural changes. So they have the ability to act as a molecular reservoir of electrons and have been used in many applications. The electrochemical behavior of the ETAB–POM SECs was also investigated by cyclic voltammetric (CV) method. As depicted in Fig. S3,† under the scan rate of 50 mV s−1, individual POM shows three reversible two-electron waves in the range from −600 to 300 mV. The wave values are different from those reported: +440, +340, and +150 mV.29 This is perhaps due to the different reference electrode used, viz. calomel electrode in this work and Ag/AgCl electrode in the literature.29 However, the CV curve of SECs presents four reversible redox couples in the potential range of −900 to 900 mV (Fig. S4†). And the peak value at about 380 mV belonging to the oxidation peak of ETAB is in agreement with the value reported early.30 Meanwhile, the characteristic peaks assigning to POM shift from −550, −390, −120 mV to −756, −496, −203 mV, respectively. The possible reason is the charge transfer between POM and ETAB molecules and the existence of long hydrophobic chains of ETAB molecule. Based on the redox property, the ETAB–POM supramolecular complex may be used in the detection of some environmental pollutants.31Conclusions
The coral-like ETAB–POM SECs were fabricated in aqueous solution by one-step synthesis method via ionic self-assembly strategy. The electrostatic interactions, π–π stacking interaction, hydrophobic interactions and van der Waals force were testified to be responsible for the preparation of the supramolecular structures. Under UV light irradiation, the coral-like morphology of SECs changed to dispersive nanospheres. This can be ascribed to the change of stacking manner and hydrophobic interaction for ETAB molecules in SECs induced by its photoisomerization behavior. Furthermore, cyclic voltammograms testify that the SECs also maintain excellent electrochemical property. The photo-responsive supramolecular materials prepared by ISA strategy in this work are expected to have potential application in phase separation, heterogeneous catalysis reaction, and detection of environmental pollutants, etc.Experimental
Materials
Phosphotungstic acid (H3[PW12O40]), 4-ethylaniline, 1,6-dibromohexane and NaNO2 were purchased from Aladdin Chemistry Co., Ltd. NaOH, HCl, ethanol, and phenol were obtained from Sinopharm Chemical Reagent Co., Ltd. All the above reagents were analytical grade and used without further purification. Triply distilled water was used to prepare aqueous solutions.Preparation
(i) In an ice bath, 50.0 ml ethanol–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution was pre-made. 4-Ethylaniline (25.0 mmol), sodium nitrite (25.0 mmol) and concentrated HCl (5.0 ml) were added in turn by stirring. A mixed solution of NaOH (25.0 mmol) and phenol (25.0 mmol) were added into the bath. The solution was stirred for 90 min, and then regulated pH ≈ 1.0. The brown precipitate was collected and washed three times by distilled water.
1) solution was pre-made. 4-Ethylaniline (25.0 mmol), sodium nitrite (25.0 mmol) and concentrated HCl (5.0 ml) were added in turn by stirring. A mixed solution of NaOH (25.0 mmol) and phenol (25.0 mmol) were added into the bath. The solution was stirred for 90 min, and then regulated pH ≈ 1.0. The brown precipitate was collected and washed three times by distilled water.
(ii) The drying precipitate (10.0 mmol) was added into 50.0 ml ethanol, then NaOH (25.0 mmol) and 1,6-dibromohexane (30.0 mmol) aqueous solutions were added. The mixture was refluxed and stirred for 8 h, and then let it stood for 24 h. The precipitates were filtrated, washed by abundant water and dried in vacuum environment for 48 h at 55 °C. The sheet-like intermediates with the color of gold yellow were obtained.
(iii) The gold yellow intermediates were added into moderate ethanol, and then trimethylamine (30% in water, 2.0 ml) was added. The reaction solution was refluxed and stirred for 120 h at 55 °C, and then cooled to room temperature. The crude product was collected by filtration and washed by cooled ethanol for several times, then dried under vacuum. The crude product was recrystallized twice from ethanol, and the final product was obtained. The chemical structure of 4-ethyl-4′-(trimethylaminohexyloxy) azobenzene bromide (ETAB) was confirmed by 1H NMR spectroscopy with a Bruker Advance 300 Spectrometer in DMSO-d6. 1H NMR (300 MHz, DMSO-d6, δ, ppm): 1.204–1.254 (t, J = 7.5 Hz, 3H, CH3), 1.309–1.404 (m, 2H, CH2), 1.457–1.555 (m, 2H, CH2), 1.666–1.839 (m, 4H, 2CH2), 2.661–2.737 (m, 2H, CH2), 3.028–3.043 (s, 9H, 3CH3), 3.256–3.327 (m, 2H, CH2), 4.076–4.118 (t, J = 6 Hz, 2H, OCH2), 7.103–7.133 (d, J = 9 Hz, 2H, 2CH), 7.398–7.426 (d, J = 8.4 Hz, 2H, 2CH), 7.761–7.788 (d, J = 8.1 Hz, 2H, 2CH), 7.857–7.887 (d, J = 9 Hz, 2H, 2CH).
Measurements
The morphologies of the ETAB–POM supramolecular assemblies were characterized by transmission electron microscopy (TEM) (JEM-100CX II (JEOL)), and the samples were prepared on carbon coated copper grids by a dip coating technique.1H NMR spectra were measured with tetramethylsilane as an internal reference at 25 °C on a Bruker AV-300 instrument. Deuterated dimenthylsulfoxide (DMSO) was selected as the solvent.
Fourier transform infrared spectroscopy (FTIR) spectra from 400 to 4000 cm−1 were measured by a VERTEX-70/70v FTIR spectrometer (Bruker Optics, Germany) on pressed thin KBr disks.
UV/vis spectra were measured in a quartz cell by using a U-4100 instrument (Hitachi, Japan). And the length of the light path is 1 mm.
Small angle X-ray scattering (SAXS) measurements were measured using an Anton-paar SAX Sess mc2 system with a Ni-filtered Cu Kα irradiation (λ = 1.5418 Å) operated at 50 kV and 40 mA. The solid samples were placed in a stainless steel tank sealed with aluminum foil. The distance from the sample to the detector was 264.5 mm.
The UV light irradiation experiments were carried out using a high-pressure mercury lamp (500 W) as the light source, and the distance from the lamp to the sample was 10 cm. Samples were exposed to air during irradiation.
Cycle voltammetric (CV) measurements were performed in a standard three-electrode cell: Saturated Calomel Electrode (SCE) as the reference electrode, a platinum plate as the counter electrode, the glassy carbon disc as the working electrode. Before each experiment, the glassy carbon electrode was polished with aluminum oxide.
Acknowledgements
This work was supported by Natural Science Foundation of China (No. 21373128), Scientific and Technological Projects of Shandong Province of China (No. 2014GSF117001), and the Natural Science Foundation of Shandong Province of China (No. ZR2011BM017).Notes and references
- C. F. Faul and M. Antonietti, Adv. Mater., 2003, 15, 673–683 CrossRef CAS.
- Y. Liu, T. Minami, R. Nishiyabu, Z. Wang and P. Anzenbacher Jr., J. Am. Chem. Soc., 2013, 135, 7705–7712 CrossRef CAS PubMed.
- Y. Zakrevskyy, J. Stumpe and C. F. J. Faul, Adv. Mater., 2006, 18, 2133–2136 CrossRef CAS.
- P. Brown, C. P. Butts and J. Eastoe, Soft Matter, 2013, 9, 2365–2374 RSC.
- Y. Wang, N. Ma, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2007, 46, 2823–2826 CrossRef CAS PubMed.
- S. Wang, N. Zhang, X. Ge, Y. Wan, X. Li, L. Yan, Y. Xia and B. Song, Soft Matter, 2014, 10, 4833–4839 RSC.
- J. F. Xu, Y. Z. Chen, D. Wu, L. Z. Wu, C. H. Tung and Q. Z. Yang, Angew. Chem., Int. Ed., 2013, 52, 9738–9742 CrossRef CAS PubMed.
- H.-i. Lee, W. Wu, J. K. Oh, L. Mueller, G. Sherwood, L. Peteanu, T. Kowalewski and K. Matyjaszewski, Angew. Chem., Int. Ed., 2007, 119, 2505–2509 CrossRef.
- Y. Yang, B. Zhang, Y. Wang, L. Yue, W. Li and L. Wu, J. Am. Chem. Soc., 2013, 135, 14500–14503 CrossRef CAS PubMed.
- H. Tian and Q. C. Wang, Chem. Soc. Rev., 2006, 35, 361–374 RSC.
- M. Lohse, K. Nowosinski, N. L. Traulsen, A. J. Achazi, L. K. von Krbek, B. Paulus, C. A. Schalley and S. Hecht, Chem. Commun., 2015, 51, 9777–9780 RSC.
- T. Yamase, Chem. Rev., 1998, 98, 307–326 CrossRef CAS PubMed.
- S. Liu, D. G. Kurth, H. Möhwald and D. Volkmer, Adv. Mater., 2002, 14, 225–228 CrossRef CAS.
- T. Zhang, S. Liu, D. G. Kurth and C. F. J. Faul, Adv. Funct. Mater., 2009, 19, 642–652 CrossRef CAS.
- P. He, B. Xu, H. Liu, S. He, F. Saleem and X. Wang, Sci. Rep., 2013, 3, 1833–1838 Search PubMed.
- J. M. Clemente-Juan, E. Coronado, J. R. Galán-Mascarós and C. J. Gómez-García, Inorg. Chem., 1999, 38, 55–63 CrossRef CAS.
- J. Xu, S. Zhao, W. Chen, M. Wang and Y. F. Song, Chem.–Eur. J., 2012, 18, 4775–4781 CrossRef CAS PubMed.
- A. Nisar, Y. Lu, J. Zhuang and X. Wang, Angew. Chem., Int. Ed., 2011, 50, 3187–3192 CrossRef CAS PubMed.
- A. Nisar, Y. Lu and X. Wang, Chem. Mater., 2010, 22, 3511–3518 CrossRef CAS.
- C. P. Pradeep, M. F. Misdrahi, F. Y. Li, J. Zhang, L. Xu, D. L. Long, T. Liu and L. Cronin, Angew. Chem., Int. Ed., 2009, 48, 8309–8313 CrossRef CAS PubMed.
- Y. Yan, H. Wang, B. Li, G. Hou, Z. Yin, L. Wu and V. W. Yam, Angew. Chem., Int. Ed., 2010, 49, 9233–9236 CrossRef CAS PubMed.
- H. Li, Y. Yang, Y. Wang, W. Li, L. Bi and L. Wu, Chem. Commun., 2010, 46, 3750–3752 RSC.
- Q. Wang, B. Zhao, X. Zhang, J. Shen and Y. Ozaki, Langmuir, 2002, 18, 9845–9852 CrossRef CAS.
- Y. Leng, J. Wang, D. Zhu, M. Zhang, P. Zhao, Z. Long and J. Huang, Green Chem., 2011, 13, 1636–1639 RSC.
- Y. Gong, Q. Hu, N. Cheng, T. Wang, W. Xu, Y. Bi and L. Yu, J. Mater. Chem. C, 2015, 3, 3273–3279 RSC.
- M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, B. Mennucci and G. Petersson, Gaussian Inc., Wallingford, CT, 2009, vol. 270, p. 271.
- Y. Gong, Q. Hu, N. Cheng, Y. Bi, W. Xu and L. Yu, RSC Adv., 2015, 5, 32435–32440 RSC.
- L. Xu, L. Feng, J. Hao and S. Dong, ACS Appl. Mater. Interfaces, 2015, 7, 8876–8885 CAS.
- Y. Wu, R. Shi, Y. L. Wu, J. M. Holcroft, Z. Liu, M. Frasconi, M. R. Wasielewski, H. Li and J. F. Stoddart, J. Am. Chem. Soc., 2015, 137, 4111–4118 CrossRef CAS PubMed.
- B. ljuki, C. E. Banks and R. G. Compton, Phys. Chem. Chem. Phys., 2004, 6, 4034–4041 RSC.
- G. Xue, J. Xiong, H. Guo, G. Cao, S. Nie and H. Hu, Electrochim. Acta, 2012, 69, 315–319 CrossRef CAS.
- T. Hayashita, T. Kurosawa, T. Miyata, K. Tanaka and M. Igawa, Colloid Polym. Sci., 1994, 272, 1611–1619 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra27351j |
| This journal is © The Royal Society of Chemistry 2016 |