Nano-NiFe2O4 catalyzed microwave assisted one-pot regioselective synthesis of novel 2-alkoxyimidazo[1,2-a]pyridines under aerobic conditions†
Soumen Payra,
Arijit Saha and
Subhash Banerjee*
and
Subhash Banerjee*
Department of Chemistry, Guru GhasidasVishwavidyalaya (A Central University), Bilaspur–495009, Chhattisgarh, India. E-mail: ocsb2006@gmail.com
First published on 25th January 2016
Abstract
Here, we have demonstrated an efficient and regioselective protocol for the synthesis of novel 2-alkoxy-3-arylimidazo[1,2-a]pyridines via microwave (MW) assisted one-pot reactions of 2-aminopyridine, β-nitrostyrenes and alcohols using nano-NiFe2O4 as a reusable catalyst under aerobic conditions. This is the first method to produce alkoxy functionalized imidazopyridine moieties furnished via sequential one-pot three component reactions. The MW irradiation expedited the reaction rate and increased the yield of products.
Functionalized imidazo[1,2-a]pyridine derivatives are well established as “privileged medicinal scaffolds” and these moieties have shown interesting biological activities such as antiviral, antitumor, antiparasitic, antimicrobial, fungicidal, anti-inflammatory, hypnotic and many more.1 These derivatives can also act as GABA and benzodiazepine receptor agonists, β-amyloid formation inhibitors, and cardiotonic agents.2 Moreover, imidazo[1,2-a]pyridine constitutes the core entity in many commercially available drugs which include alpidem, olprinone, minodronic acid (to treat anxiety, heart failure, and osteoporosis), zolimidine (peptic ulcer), necopidem, and saripidem (Fig. 1).3 Furthermore, a few of them exhibit excited-state intramolecular proton transfer.4
Accordingly different strategies have been reported to synthesize imidazo[1,2-a]pyridine scaffolds which include condensation reactions,5 oxidative coupling reactions6–9 and three-component coupling,5,10–12 intramolecular amino oxygenation13/C–H amination14 and coupling between 2-aminopyridine and nitroalkenes.15–17 However, looking inside the synthetic strategies, it was observed that most of the methods have used binucleophilic 2-aminopyridine as starting material and the initial nucleophilic addition mostly proceeds by exocyclic amino group resulting 3-nitro/carboxylic ester/keto/amino/sulfenyl18 functionalized imidazopyridine moieties. In contrast, nucleophilic addition by endocyclic pyridinium nitrogen resulting 2-functionalized imidazopyridines have rarely been observed and only 2-CO2R7b/nitro17/chloro5e substituted imidazopyridines were reported (Scheme 1).
Moreover, hydroxyl derivatives (–OH, –OR, OCOR etc.) are frequently observed in many important natural products such as betaxolol betablokker, morfin, pethidine, dextropropoxyphene, angiotensin II antagonists, tubocurarine, aspirin, pethidine, reserpine, protoaescigenin, valtrate, methysticin, vinblastine, bruceantin, michellamine B, prostratin, warfarin, fenclofenac19 and presence of these groups on any heterocycles could impart marked biological properties.19 It is obvious that introduction of alkoxy group is challenging due to the poor nucleophilicity of alcohols and as a matter of fact there is no such method available to produce alkoxy functionalized imidazopyridine moieties. Thus, invention of an efficient catalytic system to activate the poor nucleophile alcohol leading to one-pot synthesis of new class of alkoxyimidazopyridine derivatives is long-awaited.
As a part of our interest in nanocatalysis,20 here, we have observed a unique and unprecedented catalytic activity of nano-NiFe2O4 and demonstrated an efficient microwave assisted one-pot protocol for the synthesis of novel 2-alkoxy-3-arylimidazo[1,2-a]pyridine derivatives. The reactions proceed rare regioselectively via sequential aza-Michael addition, C–H imination and nucleophilic ligand transfer reaction under ambient air (Scheme 2).
 | ||
| Scheme 2 One-pot synthesis of 2-alkoxy-3-arylimidazo-[1,2-a]pyridines using nano-NiFe2O4 as catalyst. | ||
This is the first method to produce alkoxy functionalized imidazo[1,2-a]pyridine moieties. Initially, we have synthesized nano sized NiFe2O4 via sol–gel method following a previously reported protocol21 (see ESI-1 for detailed procedure†) and formation of nanoparticles was confirmed by powder X-ray diffraction (XRD), high resolution transmission electron microscopic (HRTEM), and field emission scanning electron microscopic (FESEM) studies.
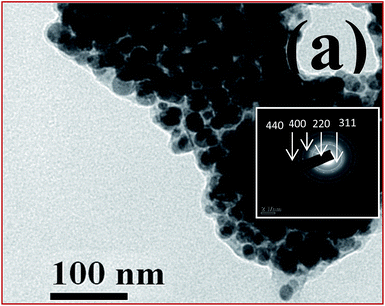
The powder XRD pattern (Fig. 2) reveals that the Bragg reflection peaks were indexed to face centered cubic NiFe2O4,22 and Fd3m space group (JCPDS file no. 10-0325) and the diffraction peaks at 30.32°, 35.81°, 37.27°, 43.45°, 53.89°, 57.41° and 62.96° are attributed to the reflections of {220}, {311}, {222}, {400}, {422}, {511} and {440} planes of the NiFe2O4, respectively. High resolution TEM image indicates the formation of spherical nano-NiFe2O4 with average size of 15 nm (Fig. 3). The selected area electron diffraction (SAED) pattern (in inset of Fig. 3) also indicates the formation of face centered cubic spinel nano-NiFe2O4 and corresponding planes {440}, {400}, {220}, {311} can be well indexed by using the SAED pattern, which is consistent with the results of XRD.
Magnified HRTEM image (Fig. 4) of NiFe2O4 nanoparticles indicate the lattice fringes with spacing of 0.20 and 0.25 nm correspond to the (400) and (311) planes of NiFe2O4, respectively. The EDAX study (Fig. 1S, ESI 2.1†) indicates that the material contains only Fe, Ni and O and no other element was observed. The FESEM image of NiFe2O4 also supported the formation of spherical particles (Fig. 2S, ESI 2.2†).
Next, using well-characterized nano-NiFe2O4 (10 mol%), we have performed the synthesis of imidazopyridines by the reaction of 2-aminopyridine (1 mmol) and (E)-1-(2-nitrovinyl)benzene (1 mmol) in ethanol. Interestingly, 2-ethoxy-3-phenylimidazo[1,2-a]pyridine (1a) was obtained (65%) after 4 h under refluxing conditions (entry 1, Table 1) via unprecedented participation of ethanol in the reaction. Inspired by the result, different reaction parameters have been examined to improve the yield and optimized the reaction conditions. The results were summarized in Table 1.
| Sl. No. | Catalyst | Time | Products | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 2-aminopyridine (1.3 mmol), (E)-1-(2-nitrovinyl)benzene (1 mmol), ethanol (2 mL) and 10 mol% of catalyst under reflux unless otherwise stated.b Isolated yields.c 2-Aminopyridine (1.0 equivalent).d 20 mol% of NiFe2O4 is used.e In DCM 2-nitro-3-phenyl-imidazopyridines was obtained.f Room temperature reactions.g Reaction was conducted using MW irradiation (70 W). | ||||
| 1 | NiFe2O4 NPs | 4 h | 1a | 59c |
| 2 | NiFe2O4 NPs | 4 h | 1a | 72 |
| 3 | NiFe2O4 NPs | 4 h | 1a | 78d |
| 4 | NiFe2O4 NPs | 4 h | — | —e |
| 5 | CuFe2O4 NPs | 4 h | 1a | 21 |
| 6 | CoFe2O4 NPs | 4 h | 1a | 22 |
| 7 | MnFe2O4 NPs | 4 h | 1a | 27 |
| 8 | Fe3O4 NPs | 4 h | 1a | 44 |
| 9 | NiO NPs (10) | 4 h | 1b | 90 |
| 10 | NiFe2O4 NPs | 15 h | 1b | 85f |
| 11 | No catalyst | 15 h | 1b | 50 |
| 12 | NiFe2O4 NPs | 5 min | 1a | 85g |
By increasing the amount of NiFe2O4 (20 mol%), no significant improvement of yield was observed (entry 3, Table 1). However, better yield (72%) was obtained using of 1.3 equivalent 2-aminopyridine (entry 2, Table 1) instead of 1 equivalent (entry 1, Table 1). When, ethanol is replaced by DCM, 2-nitroimidazopyridine was obtained (entry 4, Table 1). NiFe2O4 NPs has showed superiority over Cu–/Mn–/Co–ferrites for the synthesis of 2-ethoxyimidazopyridines. Nano-Fe3O4 catalyst yielded 44% of product (1a), whereas only NiO NPs were inefficient in producing the desired product (1a) and reaction stopped after Michael addition of endocyclic nitrogen to (E)-1-(2-nitrovinyl)benzene (1b) (entry 9, Table 1). The Michael adduct (1b) also obtained using standard conditions at room temperature (entry 10, Table 1) under catalyst-free reaction conditions. Possibly, basic Ni-sites in NiFe2O4 NP saccelerate the initial Michael addition and Fe-site promoted subsequent oxidative C–H imination and nucleophilic substitution which provides a synergistic effect for the production of unprecedented 2-ethoxy-3-phenyl-imidazopyridine (1a). Here, nano-NiFe2O4 acts as both catalyst and oxidant. In addition, use of magnetic NiFe2O4 as catalyst, added additional advantages of easy recovery by an external magnet.21 Further, NiFe2O4 nanoparticles may have exhibited extraordinary activities due to its larger and reactive surface. To improve the yield up to satisfactory level, we have conducted the reaction under microwave (MW) conditions.
It is well-known that, MW technology shortens the reaction time and improves the purity, yield and selectivity of product.23 As expected, when the same reaction was performed under MW conditions (70 W) for 5 minutes, significant improvement of yield (85%) was observed (entry 12, Table 1). Thus, the optimized yield (85%) was obtained using 1.3 equivalent of 2-aminopyridine in presence of 10 mol% NiFe2O4 NPs under MW (70 W) irradiation.
Using optimized reaction conditions i.e. 1.3 equivalents 2-aminopyridine, 10 mol% of NiFe2O4 NPs and microwave (MW, 70 W) irradiation (entry 12, Table 1), we have explored the scope of the methodology for the synthesis of library of novel 2-alkoxy-3-arylimidazopyridines. It was observed that 2-aminopyridine and β-nitrostyrene with different substituent participated in this domino reaction with primary aliphatic alcohol without any problem resulting the desired 2-alkoxy-3-arylmidazo[1,2-a]pyridine derivatives under MW irradiation. The experimental procedure is simple. Briefly, a mixture of β-nitrostyrene (1 mmol), 2-aminopyridine (1.3 mmol), ethanol (2 mL) and NiFe2O4 (10 mol%) was taken in a sealed MW tube and was irradiated MW irradiation at 70 W up to 5 minutes. After that, the mixture was cooled to room temperature and the catalyst separated by a strong external magnet and finally, product was extracted with ethyl acetate, washed with water with brine solution. The organic phase was dried over anhydrous Na2SO4. The crude product was obtained by evaporation of solvent in vacuum which was purified by column chromatography over silica gel (60–120 mesh) using mixture of petroleum ether and ethyl acetate (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as an eluting solvent to afford the pure product. The similar protocol was used for the entire product listed in Table 2.
1) as an eluting solvent to afford the pure product. The similar protocol was used for the entire product listed in Table 2.
| Sl. No. | Product | Yield (%)b | |
|---|---|---|---|
| MW/70 Wc | Heat/80 °C | ||
| a Reaction conditions: 2-aminopyridine (1.3 mmol), (E)-1-(2-nitrovinyl)benzene (1 mmol), ethanol (2 mL) and 10 mol% of NiFe2O4 NPs catalyst.b Isolated yield.c Reaction was conducted using MW irradiation (70 W). | |||
| 1 | 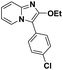 |
85 | 72 |
| 2 |  |
80 | 72 |
| 3 | 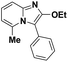 |
85 | 73 |
| 4 |  |
84 | 74 |
| 5 | 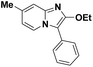 |
85 | 78 |
| 6 |  |
85 | 72 |
| 7 | 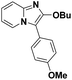 |
84 | 70 |
| 8 |  |
86 | 72 |
| 9 |  |
81 | 69 |
| 10 |  |
80 | 67 |
| 11 |  |
86 | 70 |
| 12 | 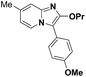 |
82 | 65 |
The results of MW assisted synthesis of 2-alkoxyimidazopyridines were also compared with conventional heating conditions (see Table 2). Different, aliphatic alcohols e.g. ethanol, n-propanol and n-butanol were participated smoothly, however, tertiary butyl alcohol and phenols were found to inactive in this reaction. This could be due to the steric hindrance at C-2 and/or poor nucleophilic nature of such substrates. Interestingly, dehydrogenation of secondary alcohol (e.g. isopropanol) took place under the similar reaction conditions and results 100% hydrogenation of β-nitrostyrene. All the imidazopyridines were properly characterized by spectroscopic (FT-IR, 1H NMR, 13C NMR etc.) studies and elemental analysis (ESI 7†). This method enables to furnish alkoxy substituted imidazopyridines. Here nano-NiFe2O4 performed showed unique action by accelerating selective aza-Michael addition and promoting oxidative imination, followed by displacing nitro group by alkoxy group resulting products. Finally, magnetic nano-NiFe2O4 has been recycled and reused for eight times without significant loss of activities (see ESI 9†).
To understand the reaction mechanism, we performed few control experiments (Scheme 3). The reaction of 2-aminopyridine and (E)-1-(2-nitrovinyl)benzene in absence of catalyst produced aza-Michael addition product (50%) under optimized reaction conditions after 15 h (eqn (1), Scheme 3).
The same reaction using NiO NPs also stopped after aza-Michael addition (90%) without further oxidative imination (eqn (1), Scheme 3). The Michael adduct underwent oxidative imination followed by nucleophilic substitution reaction in presence of NiFe2O4 NPs or Fe3O4 NPs to provide the desired product (eqn (2), Scheme 3).
These reactions indicate that Ni-site in NiFe2O4 NPs accelerates the initial aza-Michael addition and Fe-sites in ferrite promoted the oxidative addition. The oxidative addition was unsuccessful in presences of Cu-catalyst (eqn (3), Scheme 3).17 Thus, Fe-sites play crucial role in the oxidative addition reaction. The reaction in DCM produced 2-nitroimidazopyridine (eqn (4), Scheme 3) which under optimized reaction conditions does not produce 2-ethoxyimidazopyridine (eqn (5), Scheme 3). These proved that once 2-position is occupied by a group, no substitution reaction takes place to yield desired 2-ethoxyimidazopyridine product. Thus, addition of ethoxy group and displacement of –NO2 group takes place before formation of final product in an intermediate stage. A reaction was also carried out under optimized conditions in presence of 2.5 equivalent of 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), a radical quencher and 65% of the desired product was obtained which ruled out the possibility of radical pathway of this reaction. Based on the above control experiments and observations, we have proposed a plausible mechanism for the NiFe2O4 NPs catalyzed synthesis of 2-alkoy-3-aryimidazopyridine. A plausible mechanism has been outlined in Scheme 4 for 2-ethoxy-synthesis of 3-phenylimidazopyridine. The first step involves basic Ni-site of NiFe2O4 promoted aza-Michael addition of 2-aminopyridine to (E)-1-(2-nitrovinyl)benzene producing Michael adduct (I) in which the oxygen atoms of nitro group might be coordinating to Fe(III) of NiFe2O4 similar to binding of dopamine with Fe3O4 (ref. 22).
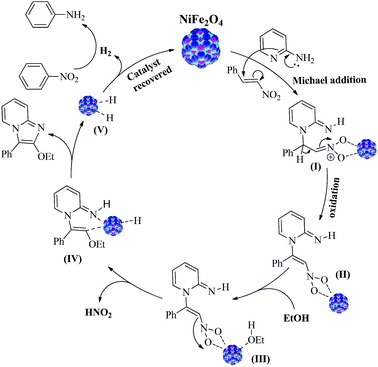 | ||
| Scheme 4 Plausible mechanism for NiFe2O4 catalyzed synthesis of 2-ethoxy-3-phenylimidazo[1,2-a]pyridine. | ||
After that, the Michael adduct (I) undergoes oxidation followed by Fe-site promoted removal of nitro group as HNO2.12 The catalyst is regenerated after H2 liberation. The elimination of H2 was proved by the reduction of nitrobenzene to aniline.
In conclusion, we have demonstrated highly regioselective microwave assisted one-pot synthesis of 2-alkoxyimidazo[1,2-a]pyridine derivatives using magnetically separable nano-NiFe2O4 as reusable catalyst. The introduction of alkoxy group is challenging due to poor nucleophilicity of alcohols and has been achieved by this nano-NiFe2O4 catalyzed domino protocol. This is the first method to synthesize alkoxy functionalized imidazopyridine moieties. Interestingly, the alkoxy group entered in a rare pathway leading to C-2 alkoxyimidazopyridine derivatives. In addition, the microwave assisted protocol improved the yields (82%) and expedited the reaction rate. Here, Ni-site of nano-NiFe2O4 accelerated selective aza-Michael addition and Fe-site promoted oxidative imination, followed by displacement of nitro group by alkoxy group. Finally, use of magnetically separable nano-NiFe2O4 as catalyst, solvent-free reaction conditions made the method environment-friendly in nature. We believe that this novel 2-alkoxyimidazo[1,2-a]pyridine derivatives will attract much attention and will find many potential biological and pharmaceutical applications.
Acknowledgements
We are pleased to acknowledge Department of Science and Technology, New Delhi, Govt. of India (No. SB/FT/CS-023/2012) for the financial support. Special thanks to Prof. B. C. Ranu and his group for their help in NMR studies.Notes and references
- M. Lhassani, O. Chavignon, J. M. Chezal, J. C. Teulade, J. P. Chapat, R. Snoeck, G. Andrei, J. Balzarini, E. D. Clercq and A. Gueiffier, Eur. J. Med. Chem., 1999, 34, 271 CrossRef CAS and the references cited therein.
- (a) A. C. Humphries, E. Gancia, M. T. Gilligan, S. Goodacre, D. Hallett, K. J. Marchant and S. R. Thomas, Bioorg. Med. Chem. Lett., 2006, 16, 1518 CrossRef CAS PubMed; (b) K. Fuchs, M. Romig, K. Mendla, H. Briem and K. Fechteler, WO2002014313, Chem. Abstr., 2002, 136, 183824r Search PubMed; (c) C. Hamdouchi, J. Blas, M. de Prado, J. del Gruber, B. A. Heinz and L. Vance, J. Med. Chem., 1999, 42, 50 CrossRef CAS PubMed.
- (a) S. Z. Langer, S. Arbilla, J. Benavides and B. Scatton, Adv. Biochem. Psychopharmacol., 1990, 46, 61 CAS; (b) K. Mizushige, T. Ueda, K. Yukiiri and H. Suzuki, Cardiovasc. Drug Rev., 2002, 20, 163 CrossRef CAS PubMed; (c) R. J. Boerner and H. Moller, Psychopharmakotherapie, 1997, 4, 145 Search PubMed.
- A. J. Stasyuk, M. Banasiewicz, M. K. Cyranski and D. T. Gryko, J. Org. Chem., 2012, 77, 5552 CrossRef CAS PubMed.
- (a) A. Gueiffier, S. Mavel, M. Lhassani, A. Elhakmaoui, R. Snoeck, G. Andrei, O. Chavignon, J.-C. Teulade, M. Witvrouw, J. Balzarini, E. D. Clercq and J.-P. Chapat, J. Med. Chem., 1998, 41, 5108 CrossRef CAS PubMed; (b) E. S. Hand and W. W. Paudler, J. Org. Chem., 1978, 43, 2900 CrossRef CAS; (c) N. Masurier, E. Moreau, C. Lartigue, V. Gaumet, J.-M. Chezal, A. Heitz, J.-C. Teulade and O. Chavignon, J. Org. Chem., 2008, 73, 5989 CrossRef CAS PubMed; (d) C. Hamdouchi, C. Sanchez and J. Ezquerra, Synthesis, 1998, 867 CrossRef CAS; (e) X. Xiao, Y. Xie, S. Bai, Y. Deng, H. Jiang and W. Zeng, Org. Lett., 2015, 17, 3998 CrossRef CAS PubMed.
- (a) A. K. Bagdi, M. Rahman, S. Santra, A. Majee and A. Hajra, Adv. Synth. Catal., 2013, 355, 1741 CrossRef CAS; (b) K. Pericherla, P. Kaswan, P. Khedar, B. Khungar, K. Parangband and A. Kumar, RSC Adv., 2013, 3, 18923 RSC; (c) D. C. Mohan, R. R. Donthiri, S. N. Rao and S. Adimurthy, Adv. Synth. Catal., 2013, 355, 2217 CrossRef; (d) Z.-J. Cai, S.-Y. Wang and S.-J. Ji, Adv. Synth. Catal., 2013, 355, 2686 CrossRef CAS.
- (a) C. He, J. Hao, H. Xu, Y. Mo, H. Liu, J. Han and A. Lei, Chem. Commun., 2012, 48, 11073 RSC; (b) J. Zeng, Y. J. Tan, M. L. Leow and X.-W. Liu, Org. Lett., 2012, 14, 4386 CrossRef CAS PubMed.
- L. Ma, X. Wang, W. Yu and B. Han, Chem. Commun., 2011, 47, 11333 RSC.
- K. Monir, A. Bagdi, S. Mishra, A. Majee and A. Hajra, Adv. Synth. Catal., 2014, 356, 1105 CrossRef CAS.
- (a) N. Chernyak and V. Gevorgyan, Angew. Chem., Int. Ed., 2010, 49, 2743 CrossRef CAS PubMed; (b) S. K. Guchhait, A. L. Chandgude and G. Priyadarshani, J. Org. Chem., 2012, 77, 4438 CrossRef CAS PubMed.
- (a) M. Adib, E. Sheikhi and N. Rezaei, Tetrahedron Lett., 2011, 52, 3191 CrossRef CAS; (b) E. F. DiMauro and J. M. Kennedy, J. Org. Chem., 2007, 72, 1013 CrossRef CAS PubMed; (c) K. Groebke, L. Weber and F. Mehlin, Synlett, 1998, 661 CrossRef CAS.
- H. Yan, Y. Wang, C. Pan, H. Zhang, S. Yang, X. Ren, J. Li and G. Huang, Eur. J. Org. Chem., 2014, 2754 CrossRef CAS.
- (a) H. Wang, Y. Wang, D. Liang, L. Liu, J. Zhang and Q. Zhu, Angew. Chem., Int. Ed., 2011, 50, 5678 CrossRef CAS PubMed; (b) D. C. Mohan, S. N. Rao and S. Adimurthy, J. Org. Chem., 2013, 78, 1266 CrossRef PubMed.
- H. Wang, Y. Wang, C. Peng, J. Zhang and Q. Zhu, J. Am. Chem. Soc., 2010, 132, 13217 CrossRef CAS PubMed.
- (a) R.-L. Yan, H. Yan, C. Ma, Z.-Y. Ren, X.-A. Gao, G.-S. Huang and Y.-M. Liang, J. Org. Chem., 2012, 77, 2024 CrossRef CAS PubMed; (b) H. Yan, R. Yan, S. Yang, X. Gao, Y. Wang, G. Huang and Y. Liang, Chem.–Asian J., 2012, 7, 2028 CrossRef CAS PubMed; (c) M.-S. Xie, Z.-L. Chu, H.-Y. Niu, G.-R. Qu and H.-M. Guo, J. Org. Chem., 2014, 79, 1093 CrossRef CAS PubMed.
- S. Santra, A. K. Bagdi, A. Majee and A. Hajra, Adv. Synth. Catal., 2013, 355, 1065 CrossRef CAS.
- K. Monir, A. K. Bagdi, M. Ghosh and A. Hajra, Org. Lett., 2014, 16, 4630 CrossRef CAS PubMed.
- C. Ravi, D. C. Mohan and S. Adimurthy, Org. Lett., 2014, 16, 2978 CrossRef CAS PubMed.
- (a) A. G. Fakim, Mol. Aspects Med., 2006, 27, 1 CrossRef PubMed; (b) A. E. Rettie, K. R. Korzekwa, K. L. Kunze, R. F. Lawrence, A. C. Eddy, T. Aoyama, H. V. Gelboin, F. J. Gonzalez and W. F. Trager, Chem. Res. Toxicol., 1992, 5, 54 CrossRef CAS PubMed; (c) A. F. Stepan, D. P. Walker, J. Bauman, D. A. Price, T. A. Baillie, A. S. Kalgutkarand and M. D. Aleo, Chem. Res. Toxicol., 2011, 24, 1345 CrossRef CAS PubMed.
- (a) S. Banerjee and S. Santra, Tetrahedron Lett., 2009, 50, 2037 CrossRef CAS; (b) S. Banerjee and G. Sereda, Tetrahedron Lett., 2009, 50, 6959 CrossRef CAS; (c) S. Banerjee, J. Das, R. Alverez and S. Santra, New J. Chem., 2010, 34, 302 RSC; (d) S. Banerjee, V. Balasanthiran, R. Koodali and G. Sereda, Org. Biomol. Chem., 2010, 8, 4316 RSC; (e) S. Banerjee, A. Horn, H. Khatri and G. Sereda, Tetrahedron Lett., 2011, 52, 1878 CrossRef CAS; (f) S. Banerjee and A. Saha, New J. Chem., 2013, 37, 4170 RSC; (g) S. Payra, A. Saha, G. Sereda and S. Banerjee, Tetrahedron Lett., 2014, 55, 5515 CrossRef; (h) A. Saha, S. Payra and S. Banerjee, Green Chem., 2015, 17, 2859 RSC; (i) S. Banerjee, New J. Chem., 2015, 39, 5350 RSC; (j) A. Saha, S. Payra and S. Banerjee, RSC Adv., 2015, 5, 101664 RSC; (k) A. Saha, S. Payra, S. K. Verma, S. Thareja and S. Banerjee, RSC Adv., 2015, 5, 100978 RSC.
- S. Shylesh, W. R. Thiel and V. Schunemann, Angew. Chem., Int. Ed., 2010, 49, 3428 CrossRef CAS PubMed.
- R. Ramesh, A. Ramanand, S. Ponnusamy and C. Muthamizhchelvan, Mater. Lett., 2011, 65, 1438 CrossRef.
- V. Polshettiwar, B. Baruwati and R. S. Varma, Green Chem., 2009, 11, 127 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedure and characterization of catalyst and 2-alkoxyimidazopyridines; copies of 1H and 13C NMR of all products listed in Table 2, reusability of NiFe2O4. See DOI: 10.1039/c5ra25540f |
| This journal is © The Royal Society of Chemistry 2016 |