A mild deposition of metallic materials on a plastic film enabled by phage display peptides†
Swathi Swaminathanc and
Yue Cui*ab
aDepartment of Electrical Engineering and Computing Systems, University of Cincinnati, Cincinnati, OH 45221, USA. E-mail: cuiy3@ucmail.uc.edu
bDepartment of Mechanical and Materials Engineering, University of Cincinnati, Cincinnati, OH 45221, USA
cDepartment of Biological Engineering, Utah State University, Logan, UT 84321, USA
First published on 21st January 2016
Abstract
We demonstrate for the first time a mild deposition of metallic materials on a plastic film, (poly)ethylene tetraphthalate (PET), enabled by phage displayed peptides. Peptide-binding motifs to PET are comprehensively screened from a library of phage displayed peptides, and a bifunctional peptide with a PET-binding motif and a metal synthesis motif is designed. This further allows for the assembly of metallic materials on PET under a mild environment.
Printing metallic-based micro/nanostructures and devices on soft substrates is of great interest for both fundamental studies and practical applications in a variety of fields, such as electronics, optics, mechanical devices, etc. Currently, metallic particle-based inks are widely used for depositing on soft substrates, followed by sintering processes at relatively high temperatures.1,2 A mild deposition of solution on soft substrates to form metallic materials has attracted great interest to different manufacturing processes, such as inkjet-printing, 3D printing, etc.
Among a variety of soft substrates, polyethylene terephthalate (PET) is widely used as a model substrate in the development of flexible sensor,3 flexible solar cell,4 flexible batteries,5 flexible transistors,6 biological scaffolds,7 magnetic devices8 and optical devices.9 PET is a thermoplastic polyester resin that has excellent mechanical flexibility and sturdiness. Previously, covalent immobilization of peptides on PET was studied, which is a non-specific chemical modification of the surface to immobilize peptides.10,11
Peptides are specific, strong biorecognition or catalytic molecules with broad chemical diversity. Specific peptides can strongly bind to their target substrates. Besides, certain peptides possess the function for catalysing the formation of metallic nanoparticles. Further, peptides can form complex, self-assembled hybrid conjugates with a variety of materials with their ability to be linked into multifunctional networks.
Phage display is a powerful bioscreening process for identifying peptide-binding motifs to specific targets.12 A library of approximately a billion (109) peptide variants is displayed on the phage as a fusion with the surface coat protein of bacteriophage, which allows for rapid, combinatorial screening of sequences displaying high affinities toward specific targets. Phage display has been investigated to identify peptides for binding to a variety of substrates, including metals,13,14 semiconductors,15–17 polymers18–20 and small molecules.21 Recently, we also have identified phage displayed peptides for the identification of graphene,22 small molecule ink,22 epoxy,20 and PDMS polymer.23,24
In this communication, we report for the first time the identification of peptide binding motifs for PET via phage display technology and the mild deposition of metallic materials on PET with the designed bifunctional peptide. The selected phage displayed peptides are shown to bind to PET surfaces. These advances allow for the design of bifunctional peptides to assemble other materials to the surface of PET to develop hybrid materials, and metallic materials have been deposited on PET surfaces.
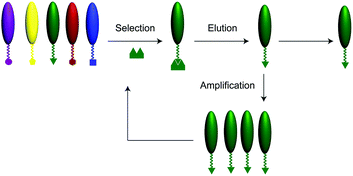
A schematic illustration of the phage display technique is shown in Fig. 1. Phage display library (New England BioLabs, Ph.D.7) of random 7-mer peptides was incubated with the PET surface (1 cm × 1 cm, Melinex ST507/200, DuPont Teijin Films, Chester, VA) in tris(hydroxymethyl)aminomethane (Tris) buffered saline containing 0.1–0.5% Tween-20 (TBST) for 1 h at room temperature. The PET film was then washed several times with TBST buffer. Then the phages were eluted from the film by addition of glycine–HCl (pH 2.2) for 15 min, neutralized with Tris–HCl, pH 9.1, to collect the bound phage molecules. Eluted phages were then amplified in E. coli, and the panning was repeated for up to three rounds, under increasingly stringent conditions, to obtain phage clones expressing peptides having the highest binding affinities to the PET samples. After the final round of panning, DNA sequence analysis of the isolated phage clones yielded heptameric PET-binding peptides.
Table 1 shows the phage displayed peptide sequences for binding to PET and their properties. Two of the peptides DEYCCNN and NALVQIS were found to bind to the PET surface more frequently, with the frequencies of 3/25 and 2/25 respectively, compared to other peptides. As shown in Table 1, the natures of the peptides (with C-terminus amidated to avoid the free charge) were analyzed, including PI, net charge, average hydrophilicity, and ratio of hydrophilic residues to total number of residues. From the table, the PET-binding peptides exhibit a strong inclination towards hydrophobicity, which is evident from the low average hydrophilicity ratios for these peptides. The isoelectric points and their corresponding net charge at pH 7.0 have also been analyzed in Table 1. Majority of the peptide sequences carry no net charge/net negative charge/low net positive charge at neutral pH and also exhibit high values of pI.
| Num | Freq | Sequence | Hydrophilic ratio (%) | pI | Charge at pH 7 |
|---|---|---|---|---|---|
| 1 | 3/25 | DEYCCNN | 29 | 4 | −2 |
| 2 | 1/25 | IPYDNNQ | 14 | 5.4 | −1 |
| 3 | 1/25 | AIVGTPF | 0 | 7.8 | 0 |
| 4 | 1/25 | TIMEYRL | 29 | 7.8 | 0 |
| 5 | 1/25 | DEVKTIA | 43 | 5.7 | −1 |
| 6 | 1/25 | ITLHMGM | 14 | 7.9 | 1 |
| 7 | 1/25 | HVPHPLL | 29 | 7.93 | 2 |
| 8 | 2/25 | NALVQIS | 0 | 7.8 | 0 |
| 9 | 1/25 | MGTNSP | 0 | 7.8 | 0 |
| 10 | 1/25 | YVATEIT | 14 | 5.6 | −1 |
| 11 | 1/25 | YPGGYSR | 14 | 9.2 | 1 |
| 12 | 1/25 | HVTSVQT | 14 | 7.9 | 1 |
| 13 | 1/25 | ELLASPW | 14 | 5.5 | −1 |
| 14 | 1/25 | YMGPASN | 0 | 7.8 | 0 |
| 15 | 1/25 | HAMRAQP | 29 | 10.6 | 2 |
| 16 | 1/25 | TTNLDKA | 29 | 7.8 | 0 |
| 17 | 2/25 | LSNNNLR | 14 | 10.6 | 1 |
| 18 | 1/25 | SINAATS | 0 | 7.8 | 0 |
| 19 | 1/25 | IDFTGAT | 14 | 5.5 | −1 |
| 20 | 1/25 | SYTTVVQ | 0 | 7.8 | 0 |
| 21 | 1/25 | FDTLKIL | 29 | 7.8 | 0 |
Fluorescent characterization of the binding of phage-displayed peptides to PET was studied. This was accomplished by incubating the PET substrates (1 cm × 1 cm) sequentially to (1) amplified single-colony phage displayed peptides, DEYCCNN, NALVQIS, or AIVGTPF, (2) blocking buffer (0.1 M NaHCO3, 1% BSA, at pH 8.6), (3) anti-M13 phage coat protein–biotin conjugated monoclonal antibody, and (4) avidin–fluorescein isothiocyanate (FITC), with buffer washing steps in between to remove non-specific binding. The colour intensity on the PET surface was observed with a fluorescence microscope. As shown in Fig. 2, clear fluorescent signals were observed for PET substrates via incubation with PET-binding phage displayed peptides. A control experiment was also conducted with M13 phage without phage-displayed peptides, with the above procedure. The absence of fluorescent signals on PET surface can be correlated to the absence of phage-displayed peptide in M13 phage. This indicates that the PET-binding phage displayed peptides are essential for generating fluorescent signals.
 | ||
| Fig. 2 Fluorescent characterization of phage displayed peptides on PET. (a) DEYCCNN, (b) NALVQIS, (c) AIVGTPF, and (d) M13 phage without phage displayed peptide. Scale bar: 50 μm. | ||
Next, the recognition of PET-binding phage displayed peptides to micropatterned PET was studied. Photolithography was conducted on PET with photoresist AZ5214. Photomasks with different dimensions of line and boxes were used for generating different micropatterns of photoresists on PET. Fluorescent characterization for the binding of these phage molecules to micropatterned PET surfaces was investigated, as shown in Fig. 3. The photoresist-patterned PET film was exposed to PET-binding phage-displayed peptides (DEYCCNN), blocking buffer, anti-M13 antibody, and avidin–FITC with washing steps in between. By introducing appropriate phage displayed peptides, the phages bound to the specific locations mapped by the photoresist. Thus, the fluorescent signals were patterned on the PET substrates due to presence of patterned AZ5214 on PET substrates.
 | ||
| Fig. 3 Fluorescent characterization of phage displayed peptides on PET with different patterns of photoresist (a–d). Scale bar: 50 μm. | ||
Further, the assembly of metallic materials on PET was studied. A bifunctional peptide (DEYCCNN-GGGG-NPSSLFRYLPSD) was designed and synthesized. It has the PET-binding motif (DEYCCNN) screened from a phage display library and a silver (Ag) synthesis motif (NPSSLFRYLPSD).24,25 A PET substrate (1 cm × 1 cm) was incubated with the bifunctional peptides (10 μl, 50 mM) to immobilize the peptides on PET. The bifunctional peptide-immobilized PET was incubated with 30 mM silver nitrate for 3 days, which resulted in the growth of Ag nanoparticles on the PET surface. Fig. 4 shows the SEM and EDX characterization of the synthesis of Ag nanoparticles on the PET surface in the presence of bifunctional peptides and in the absence of bifunctional peptides on PET surface. The SEM image (Fig. 4a left) shows the Ag nanoparticles were synthesized effectively from silver nitrate solution when there are bifunctional peptides immobilized on PET. While in the absence of bifunctional peptides, though there was silver nitrate solution, there was no Ag nanoparticle on the surface. From the EDX characterization, an Ag percentage of over 70% was obtained for the bifunctional peptide-immobilized PET surface (Fig. 4b). While in the absence of bifunctional peptides (Fig. 4c), it shows no Ag was identified. The results indicate that the bifunctional peptides are essential for the synthesis and deposition of metallic materials on a plastic film under a mild condition.
Conclusions
We have demonstrated for the first time the identification of peptide-based binding motifs to PET from a phage display library, and the assembly of metallic materials on PET with designed bifunctional peptides under a mild condition. The approach we describe here may open new avenues for a variety of fundamental studies of the properties of plastic substrates and the practical applications in 3-D printing, inkjet printing, flexible electronics, flexible optics, biomaterials, and surface and interface, etc. Although these results are promising, further studies are needed to obtain fully conductive plastic substrates with the mild deposition of metallic materials via phage-displayed peptides.Acknowledgements
The authors greatly acknowledge Fenn-Ann Shen for assistant in SEM characterization.References
- A. Kamyshny, M. Ben-Moshe, S. Aviezer and S. Magdassi, Macromol. Rapid Commun., 2005, 26, 281–288 CrossRef CAS.
- K. C. Yung, S. P. Wu and H. Liem, J. Mater. Sci., 2009, 44, 154–159 CrossRef CAS.
- P. Labroo and Y. Cui, Biosens. Bioelectron., 2013, 41, 852–856 CrossRef CAS PubMed.
- Y. Gong, C. H. Li, X. M. Huang, Y. H. Luo, D. M. Li, Q. B. Meng and B. B. Iversen, ACS Appl. Mater. Interfaces, 2013, 5, 795–800 CAS.
- G. Socol, M. Socol, N. Stefan, E. Axente, G. Popescu-Pelin, D. Craciun, L. Duta, C. N. Mihailescu, I. N. Mihailescu, A. Stanculescu, D. Visan, V. Sava, A. C. Galca, C. R. Luculescu and V. Craciun, Appl. Surf. Sci., 2012, 260, 42–46 CrossRef CAS.
- H. Y. Lee, W. Y. Ye, Y. H. Lin and C. T. Lee, J. Disp. Technol., 2014, 10, 792–796 CrossRef CAS.
- H. Mirzadeh, E. V. Moghadam and H. Mivehchi, J. Biomed. Mater. Res., Part A, 2011, 98, 63–71 CrossRef PubMed.
- Y. Cao and C. G. Zhou, J. Magn. Magn. Mater., 2012, 324, 1832–1836 CrossRef CAS.
- C. S. Oh, S. M. Lee, E. H. Kim, E. W. Lee and L. S. Park, Mol. Cryst. Liq. Cryst., 2012, 568, 32–37 CrossRef CAS.
- Y. Lei, M. Remy, C. Labrugere and M. C. Durrieu, J. Mater. Sci.: Mater. Med., 2012, 23, 2761–2772 CrossRef CAS PubMed.
- S. Kakinoki and T. Yamaoka, Bioconjugate Chem., 2015, 26, 639–644 CrossRef CAS PubMed.
- L. Stryer, Biochemistry, W.H. Freeman & Company, New York, 4th edn, 1995 Search PubMed.
- R. J. Zuo, D. Ornek and T. K. Wood, Appl. Microbiol. Biotechnol., 2005, 68, 505–509 CrossRef CAS PubMed.
- R. R. Naik, S. J. Stringer, G. Agarwal, S. E. Jones and M. O. Stone, Nat. Mater., 2002, 1, 169–172 CrossRef CAS PubMed.
- E. Estephan, M. B. Saab, C. Larroque, M. Martin, F. Olsson, S. Lourdudoss and C. Gergely, J. Colloid Interface Sci., 2009, 337, 358–363 CrossRef CAS PubMed.
- C. B. Mao, D. J. Solis, B. D. Reiss, S. T. Kottmann, R. Y. Sweeney, A. Hayhurst, G. Georgiou, B. Iverson and A. M. Belcher, Science, 2004, 303, 213–217 CrossRef CAS PubMed.
- E. Estephan, M. B. Saab, M. Martin, C. Larroque, F. J. G. Cuisinier, O. Briot, S. Ruffenach, M. Moret and C. Gergely, J. Pept. Sci., 2011, 17, 143–147 CrossRef CAS PubMed.
- M. Vodnik, B. Strukelj and M. Lunder, Anal. Biochem., 2012, 424, 83–86 CrossRef CAS PubMed.
- A. B. Sanghvi, K. P. H. Miller, A. M. Belcher and C. E. Schmidt, Nat. Mater., 2005, 4, 496–502 CrossRef CAS PubMed.
- S. Swaminathan and Y. Cui, Mater. Sci. Eng., C, 2013, 33, 3082–3084 CrossRef CAS PubMed.
- F. Sugawara, J. Pestic. Sci., 2004, 29, 279–283 CrossRef.
- Y. Cui, S. N. Kim, S. E. Jones, L. L. Wissler, R. R. Naik and M. C. McAlpine, Nano Lett., 2010, 10, 4559–4565 CrossRef CAS PubMed.
- S. Swaminathan and Y. Cui, RSC Adv., 2012, 2, 12724–12727 RSC.
- S. Swaminathan, M. Bullough, Q. Li, A. Zhou and Y. Cui, J. R. Soc., Interface, 2014, 11, 20130893 CrossRef PubMed.
- Z. Xu, Y. Peng, Y. Wantai and C. Jinchun, Mater. Sci. Eng., C, 2008, 28, 237–242 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra25429a |
| This journal is © The Royal Society of Chemistry 2016 |