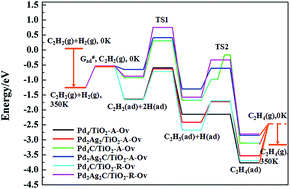
It is well known that the carbon species has a strong effect on the catalytic activity and selectivity of acetylene (C2H2) hydrogenation, and the previous theoretical work has mainly concentrated on the catalyst without the support, so it is important to investigate the carbon species effect on the behavior of selective hydrogenation over the metal oxide supported palladium (Pd)-based catalysts. In this work, the effect of the carbon atom on C2H2 hydrogenation is studied on the oxygen-defective anatase (TiO2-A-Ov) and rutile (TiO2-R-Ov) supported Pd4 (Pd2Ag2) clusters using density functional theory calculations with a Hubbard U correction. The calculated results show that the carbon atom has a large effect on both catalytic and selectivity of C2H2 hydrogenation, and that such a promotion effect is strongly related to the properties of the support, i.e., the carbon species effect is different for anatase TiO2 and rutile TiO2. By analysing the hydrogenation reaction mechanism, it was found that: (i) the subsurface carbon atom can improve the selectivity of ethylene on the rutile supported PdAg metal cluster, but has little effect on its activity, (ii) in contrast to the rutile support, the presence of carbon species can enhance the activity of the anatase supported PdAg metal cluster, but has a little effect on its selectivity, (iii) the addition of Ag can further increase the selectivity, and (iv) the TiO2 support can enhance the selectivity when compared to the catalyst without the TiO2 support.