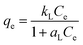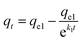DOI:
10.1039/C5RA25247D
(Paper)
RSC Adv., 2016,
6, 11522-11527
Adsorption of polygalacturonic acid on crosslinked polystyrene spheres with cationic polyelectrolyte
Received
27th November 2015
, Accepted 11th January 2016
First published on 14th January 2016
Abstract
In a papermaking system, severe drawbacks on machine runnability and paper quality occur due to a build-up of dissolved and colloidal substances (DCS) in the whitewater. The adsorption of DCS on solid sorbents is proposed to remove DCS from the whitewater. Polystyrene (PS) spheres with poly(methacryloxyethyltrimethyl ammonium chloride) (poly-MAC) were prepared as adsorbents for the removal of polygalacturonic acid (PGA) from aqueous solution. Characterization of these particles was carried out using Fourier transform infrared spectroscopy, 13C NMR, scanning electron microscopy, particle size analyzer, surface area apparatus and elemental analyzer. The results indicate that the cationic polyelectrolyte was grafted onto the surface of PS spheres. The maximum adsorption capacity of the cationic PS spheres for PGA was found to be 123.07 mg g−1. Adsorption of PGA on the these sorbents all fitted to the Langmuir model and followed pseudo-first-order kinetics. Adsorption capacity of PGA on the cationic PS spheres was higher than on IRA-67 (alkaline ion exchange resin). In the removal contaminants from whitewater using the adsorption method, it is a promising route to graft functional polymers on the solid matrixes, resulting in high performance.
1. Introduction
Dissolved and colloidal substances (DCS) in a papermaking system continuously accumulate with increasing utilization of recycled whitewater. Besides insoluble hydrophobic substances, there are a variety of dissolved anionic substances mainly coming from pectin, hemicelluloses, oxidized starch, carboxyalkyl cellulose, etc.1,2 Accumulation of these substances leads to many serious problems, such as poor runnability of the paper machine, inefficiency of chemicals, and poor physical properties of the paper produced.3–5 DCS are generally anionic in character and are referred to as “anionic trash” because they can form polyelectrolyte complexes with added cationic chemicals.
Current available technologies to control the detrimental substances are mostly based on chemical treatments. The use of highly cationic polymers is a commonly considered possibility to remove DCS by fixing them onto the fibers.6,7 However, the fixed DCS in the fiber mat may still impair machine runnability, especially in the press and drying sections.8 Other treatment technologies, such as biological enzymes,9,10 membrane filtration treatment,11,12 dissolved air flotation and induced air flotation,13,14 have been proposed, but adaptability and costs have restricted implementations in mills.
Adsorption of contaminants from process water using suitable adsorbents in a fluidized bed reactor has been proposed to allow papermaking operations under highly cycled whitewater operations. Regarding ion-exchange resins, it is known that polystyrene (PS) spheres are widely used as the matrix material because they are cheap, accessible and have high mechanical strength.15 The properties of ion-exchange resins mainly depend on the architecture of the surface functional groups.16,17 Fibrous polymers, such as quaternary ammonium polyelectrolyte anchored to PS spheres, might exhibit exciting adsorption behavior. In such a structure, the flexibility and partial mobility of the polyelectrolyte are expected to provide rapid interaction with the target molecules.
Polygalacturonic acid (PGA) is a polysaccharide composed of galacturonic acid units and is the main source of DCS from mechanical pulp.18,19 After peroxide bleaching, more PGA can be released from the pulp suspension, increasing the cationic demand of the whitewater.20,21 Studies have shown that PGA not only can consume chemical agents, but also combine with calcium or magnesium ions, leading to insoluble deposits.22,23 Whatever its form of existence, PGA can be adsorbed by physical effects or electrostatic attraction using a polymeric adsorbent. Hence, PGA was selected as a model contaminant, and PS spheres were used as the adsorbent in this study. PS spheres were firstly acylated by acryloyl chloride and then grafted with poly(methacryloxyethyltrimethyl ammonium chloride) (poly-MAC) using surface-initiated free-radical polymerization. The obtained products were characterized by infrared spectra, 13C NMR, scanning electron microscopy, particle size analyzer, surface area apparatus and elemental analyzer. Finally, the effect of initial PGA concentration, contact time, temperature, NaCl concentration, and sorbent recycling were investigated.
2. Experimental
2.1 Materials
Dichloromethane was dried with calcium hydride (CaH2) overnight and distilled before use. Ammonium persulfate, anhydrous ethanol, sulfuric acid, sodium chloride, and carbazole were of analytical grade and used without purification. IRA-67 (ion exchange resin), acryloyl chloride and methacryloxyethyltrimethyl ammonium chloride (MAC) were obtained from J&K Scientific Co., Ltd. Polygalacturonic acid (PGA) was purchased from Aladdin Industrial Corporation with a molecular weight between 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 50
000 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. PGA was purified from orange to a purity higher than 90%. The stock solution was made by dissolving PGA in 0.1 M NaOH over 24 h. This solution was then adjusted to pH 7 with HCl. Crosslinked polystyrene spheres (PS spheres) were kindly supplied by Zhejiang Zhengguang Industrial Co., Ltd., China as shown in Table 1.
000. PGA was purified from orange to a purity higher than 90%. The stock solution was made by dissolving PGA in 0.1 M NaOH over 24 h. This solution was then adjusted to pH 7 with HCl. Crosslinked polystyrene spheres (PS spheres) were kindly supplied by Zhejiang Zhengguang Industrial Co., Ltd., China as shown in Table 1.
Table 1 Physical and chemical properties of PS spheres and IRA-67 resin
| |
Matrix |
Average particle size (μm) |
Specific surface area (m2 g−1) |
Pore volume (cm3 g−1) |
Degree of crosslinking |
Functional group |
Poly-MAC loading (mmol g−1) |
| PS-a |
Styrene–DVB |
250 |
35.5 |
0.28 |
7% |
— |
— |
| PS-b |
Styrene–DVB |
400 |
36.6 |
0.26 |
7% |
— |
— |
| PS-c |
Styrene–DVB |
200 |
57.5 |
0.42 |
15% |
— |
— |
| CPS-a |
Styrene–DVB |
390 |
— |
— |
— |
Quaternary-N |
1.44 |
| CPS-b |
Styrene–DVB |
680 |
— |
— |
— |
Quaternary-N |
1.09 |
| CPS-c |
Styrene–DVB |
240 |
— |
— |
— |
Quaternary-N |
1.12 |
| IRA-67 |
Acrylic–DVB |
630 |
— |
— |
— |
Tertiary-N |
— |
2.2 Preparation of PS spheres with cationic polyelectrolyte brushes
PS spheres were firstly purified by tetrahydrofuran, distilled water, and ethanol prior to use. In an efficient fume cupboard, 0.26 g of purified PS spheres was placed in a 50 mL flask equipped with a CaCl2 guard tube. 5 g of anhydrous dichloromethane was added, and the mixture was magnetically stirred for 1 hour. After adding 0.68 g of acryloyl chloride, 0.73 g of anhydrous aluminium chloride was batch-wise added with continuous stirring. The reaction solution was stirred at 30 °C for 4 h and then thrown into a substantial amount of distilled water to quench the reaction. Finally, the crude product was successively washed with ethanol, dilute hydrochloric acid (volume ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) and distilled water for 30 min.
100) and distilled water for 30 min.
Acylated PS spheres grafted with poly-MAC were synthesized in a 250 mL three-necked flask. 0.2 g of acylated PS spheres were suspended in 8.1 g of N,N-dimethyl formamide and swelled for 12 h. After this period, the initiator ammonium persulfate (APS, 3 mg) and MAC monomer (4 g) were charged into the flask with continuous stirring at 60 °C for 4 h. Then the reaction was continued at 80 °C for 3 h. At the end, the mixture was cooled, filtered, washed with a large amount of distilled water and methanol (50 mL), and dried under vacuum at 50 °C for 24 h. The products were still in spherical form and bigger than the initial size, as shown in Table 1.
2.3 Characterizations
The Fourier transform infrared (FT-IR) spectra were carried out on a spectrophotometer (Thermo Nicolet, NEXUS 670, USA) at room temperature, and the samples were prepared in pellet form using KBr. The surface morphologies of PS spheres were observed using scanning electron microscope (ZEISS, EVO18, Germany). The 13C solid-state NMR (600 MHz) spectra were obtained with a Bruker plus-600 spectrometer (Bruker, Germany). The average particle sizes of cationic PS spheres were measured by a laser particle size analyzer (Malvern, Mastersizer 3000, Britain). The specific surface area and core volume of PS spheres were determined by using a surface area apparatus (Micromeritics, ASAP2020, USA). Element analyses of cationic PS spheres were investigated by elemental analyzer (Elementar, Vario EL cube, Germany), and polyelectrolyte loading was calculated by the following equation:| |
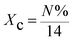 | (1) |
where Xc (mmol g−1) is the polyelectrolyte loading of PS spheres, N% is nitrogen content of cationic PS spheres, and 14 is nitrogen relative molecular mass.
2.4 Adsorption experiments
The adsorption of PGA was investigated using batch technique in a 50 mL centrifuge tube at different temperatures. 0.1 g of modified PS spheres or IRA-67 resin were dispersed in 20 mL solutions with different PGA concentrations. The effect of initial concentration of PGA, adsorption time and inorganic salt concentration under neutral conditions were studied. The supernatant in PGA-containing samples was analyzed by comparing the absorbance before and after adsorption with an ultraviolet spectrophotometer (DR5000, Hatch Company, USA) at 530 nm.24 The amount of PGA, x (mg L−1), was calculated according to the equation (y = 0.0091x − 0.0012, R2 = 0.9922). The recycling capabilities of cationic PS spheres and IRA-67 resin were evaluated by repeating adsorption/elution experiments. The absorbents were rinsed with 1 mol L−1 sodium hydroxide and 1 mol L−1 hydrochloric acid, successively. This regeneration process was carried out at room temperature for 10 min.
3. Results and discussion
3.1 Characteristics of the PS spheres with cationic polyelectrolyte
The FT-IR spectroscopy method was used to obtain information on the functional groups of the PS spheres. The spectra of PS and modified PS are presented in Fig. 1. From all the spectra, the absorption band at 826 cm−1 was assigned to the hydrocarbon bond (C–H) bending vibration of the benzene ring, respectively. For the acrylated PS, the band at 1681 cm−1 corresponded to the stretching vibration of the carbonyl group (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). The spectrum of cationic PS exhibits some differences such as: (1) the presence of the absorption band at 1726 cm−1, which corresponds to the ester carbonyl group (O
O). The spectrum of cationic PS exhibits some differences such as: (1) the presence of the absorption band at 1726 cm−1, which corresponds to the ester carbonyl group (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O) stretching vibration; (2) appearance of the absorption band at 954 cm−1 belonging to the quaternary ammonium salt from the MAC unit.
C–O) stretching vibration; (2) appearance of the absorption band at 954 cm−1 belonging to the quaternary ammonium salt from the MAC unit.
 |
| | Fig. 1 FT-IR spectra of PS spheres (a: non-modified PS spheres, b: acylated PS spheres, c: cationic PS spheres). | |
The 13C solid-state NMR spectra of PS and modified PS are shown in Fig. 2. From Fig. 2a, the resonances with chemical shifts of about 40.2 ppm and 45.9 ppm can be assigned to CH2 and CH units in the PS backbone, respectively. In Fig. 2b, a new peak of about 193.0 ppm can be assigned to ketone carbonyl, indicating that acryloyl chloride had been bonded onto the PS. In Fig. 2c, there were some new resonances with chemical shifts of about 192.9 ppm, 173.5 ppm, 62.4 ppm, 57.6 ppm, 52.30 ppm, 43.0 ppm, 38.4 ppm, and 17.7 ppm, which were associated with ketone carbonyl, ester carbonyl, –CH2–O–, quaternary carbon, –CH2–N–, –CH, –CH2–, and –CH3. These indicate that poly-MAC had been grafted onto the PS spheres.
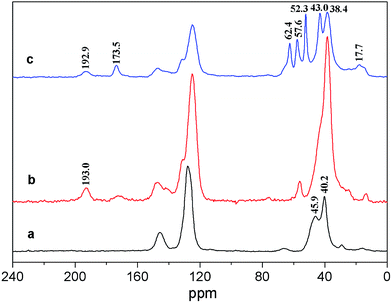 |
| | Fig. 2 13C-NMR spectra of PS spheres (a: non-modified PS spheres, b: acylated PS spheres, c: cationic PS spheres). | |
SEM images of unmodified and modified PS spheres are displayed in Fig. 3. It can be seen that the surfaces of unmodified PS spheres (Fig. 3a–c) were smooth. The average particle sizes of PS-a, PS-b and PS-c were 250 μm, 400 μm and 200 μm, respectively. After grafting the cationic polyelectrolyte, the modified PS spheres (Fig. 3d–f) became rougher and larger. The average particle size of CPS-a, CPS-b and CPS-c grew to 390 μm, 680 μm and 240 μm, respectively. These results indicate that the poly-MAC was successfully grafted on the surface of PS spheres.
 |
| | Fig. 3 SEM of PS spheres (a–c: PS spheres PS-a, PS-b, PS-c. d–f: cationic PS spheres CPS-a, CPS-b, CPS-c). | |
The results of the modified PS spheres are given in Table 1. The matrix of CPS-a, CPS-b and CPS-c were all styrene–DVB copolymer, while IRA-67 was acrylic–DVB copolymer. In order to exchange with anionic ions, tertiary amine molecules were anchored on the IRA-67 as functional groups. Poly-MAC were grafted on the surface of CPS-a, CPS-b and CPS-c with functional groups of quaternary ammonium salts. The poly-MAC loaded on the surface of CPS-a, CPS-b and CPS-c were 1.44 mmol g−1, 1.09 mmol g−1 and 1.12 mmol g−1, respectively.
3.2 Effect of initial PGA concentration on adsorption
The equilibrium adsorption isotherms are fundamental in describing the interaction between the adsorbent and adsorbate. First of all, the effect of initial PGA concentration on adsorption was studied, and the isothermal curves are presented in Fig. 4. According to the adsorption data, two common isotherm models for adsorption were used to fit the experimental data in Table 2. The Langmuir isotherm model (eqn (2)) and Freundlich isotherm model (eqn (3)) are as follows:| |
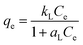 | (2) |
where qe (mg g−1) represents the amount of material adsorbed at adsorption equilibrium, and Ce is the equilibrium adsorbent concentration. kL, aL, aF and bF are the corresponding parameters for isotherms.
 |
| | Fig. 4 Adsorption isotherms of PGA onto the different adsorbents (adsorbent amount: 5 g L−1, temperature: 60 °C, reaction time: 60 min). | |
Table 2 Isotherm parameters of PGA adsorption onto different adsorbents
| Resin samples |
Langmuir model |
Freundlich model |
| kL |
aL |
R2 |
aF |
bF |
R2 |
| CPS-a |
0.217 |
0.00069 |
0.9905 |
0.6458 |
0.7702 |
0.9805 |
| CPS-b |
0.2732 |
0.00392 |
0.9629 |
2.7529 |
0.4437 |
0.8806 |
| CPS-c |
0.3089 |
0.00658 |
0.9453 |
4.0364 |
0.3433 |
0.8084 |
| IRA-67 |
0.3528 |
0.00845 |
0.8807 |
4.9866 |
0.2997 |
0.7080 |
In Table 2, R2 values referring to the Langmuir model of CPS-a, CPS-b, CPS-c and IRA-67 were 0.9905, 0.9629, 0.9453, and 0.8807, respectively. Meanwhile, R2 values referring to the Freundlich model of CPS-a, CPS-b, CPS-c and IRA-67 were 0.9805, 0.8806, 0.8084, 0.7080, respectively. These R2 values indicate that isothermal adsorption of PGA on the resins occurred mainly according to the Langmuir adsorption model. After 60 minutes, the saturated adsorption amount of PGA on the CPS-a, CPS-b, CPS-c and IRA-67 were 123.07 mg g−1, 53.61 mg g−1, 38.87 mg g−1, and 35.88 mg g−1, respectively. As for the surface molecular form of these adsorbents, a lot of tertiary amine groups anchored on the surface of the gel-type ion exchange resin (IRA-67), while polymer chains of the cationic polyelectrolyte were grafted on the other adsorbents. In theory, the flexible cationic polyelectrolyte could catch more PGA molecules than the anchored, rigid tertiary amine groups. Among these cationic polystyrene spheres, the more cationic polyelectrolytes were grafted on the surface of adsorbents, the less PGA molecules existed in the solutions.
3.3 Effect of contact time on adsorption
The effect of contact time on the amount of PGA adsorbed onto adsorbent materials was studied, and the adsorption kinetics curves are presented in Fig. 5. According to the adsorption data, two common kinetic models for adsorption were used to fit the experimental data in Table 3. The pseudo-first-order model (eqn (4)) and intraparticle diffusion model (eqn (5)) are illustrated as follows:| |
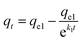 | (4) |
where qt (mg g−1) is the amount of PGA at time t (min), and qe1 and k1 are the parameters for the kinetics of pseudo-first-order. kid and C are parameters for the kinetics of intraparticle diffusion.
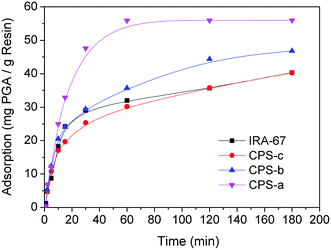 |
| | Fig. 5 Adsorption kinetics of PGA onto the different adsorbents (PGA concentration: 300 mg L−1, adsorbent amount: 5 g L−1, temperature: 60 °C). | |
Table 3 Kinetic parameters of PGA adsorption onto different adsorbents
| Resin samples |
Pseudo-first-order |
Intraparticle diffusion |
| qe1 (mg g−1) |
k1 (min−1) |
R2 |
C (mg g−1) |
kid (mg (g min0.5)−1) |
R2 |
| CPS-a |
56.6767 |
0.05756 |
0.9956 |
7.54312 |
4.56123 |
0.7590 |
| CPS-b |
42.9915 |
0.05036 |
0.9539 |
4.73951 |
3.57653 |
0.8904 |
| CPS-c |
35.92755 |
0.05127 |
0.9521 |
4.11561 |
2.98581 |
0.8971 |
| IRA-67 |
36.21749 |
0.06394 |
0.9709 |
5.36004 |
2.96370 |
0.8225 |
The theoretical kinetic parameters, qel, kl, C, kid and R2, are listed in Table 3. For PGA, the R2 value for the pseudo-first-order model was much closer than that of the intraparticle diffusion model. In Fig. 5, the curves show that the PGA adsorption amount increased rapidly in the first 15 min, in the following order of adsorption rate of the adsorbents: CPS-a > CPs-b ≈ IRA-67 > CPS-c. These adsorbents not only have functional groups on the surfaces, but also in the channels. The more functional groups there are, the better the adsorption performance. The CPS-a, with 1.44 mmol g−1 cationic polyelectrolyte, had the fastest adsorption rate in the first 15 min, following pseudo-first-order kinetics. At the same amount of graft, cationic polyelectrolytes on the surface of spheres more easily catch PGA than do polyelectrolytes in the channels. PS-c has bigger pore volume than PS-b, so there was less cationic polyelectrolyte on the surface of CPS-c than CPS-b. Adsorption rate of CPS-b was faster than CPS-c.
3.4 Effect of NaCl concentration on adsorption
In the whitewater, there are a large number of sodium ions, calcium ions, magnesium ions and other metal ions. Sodium chloride is one of the most salts in the whitewater. Fig. 6 shows that NaCl influenced the adsorption capacity of IRA-67 for PGA, but little influenced that of CPS-a. PGA adsorption capacity on CPS-a slightly reduced from 56.94 mg PGA/g to 55.84 mg PGA/g with the increase of NaCl concentration from 0 to 0.1 mol L−1. However, the PGA adsorption capacity of IRA-67 dropped from 35.31 mg PGA/g to 1.84 mg PGA/g with the increase of NaCl concentration from 0 to 0.1 mol L−1. The molecular conformation transformed with the concentration of sodium chloride. The radius of gyration of PGA became smaller with the increase of the salt concentration. Such that for IRA-67, the coagulating PGA in the sodium chloride solution was too hard to be caught by tertiary amine molecules with a short chain. Regarding CPS-a, a large number of flexible cationic polyelectrolytes on the surface might not be blocked by metal ions, and thus they easily captured the coagulating PGA. Therefore, the adsorption capacity of CPS-a for PGA was maintained at a high level regardless of the concentration of sodium chloride.
 |
| | Fig. 6 Effect of NaCl concentration on the adsorption of PGA (PGA concentration: 300 mg L−1, adsorbent amount: 5 g L−1, temperature: 60 °C, reaction time: 60 min). | |
3.5 Sorbent recycling
The economic feasibility of using adsorbent to remove contaminants from whitewater relies on its reusability for multiple adsorption/desorption cycles. In this study, the optimal desorption condition for PGA was 1 M HCl. As seen from Fig. 7, CPS-a and IRA-67 were used for six cycles. With the increasing number of cycles, the PGA adsorption capacities of CPS-a and IRA-67 were reduced. After six cycles, adsorption capacities of CPS-a and IRA-67 were reduced from 56.41 mg PGA/g and 33.49 mg PGA/g to 47.75 mg PGA/g and 26.74 mg PGA/g, respectively. The total amount of PGA adsorbed on the CPS-a and IRA-67 had no significant decrease in six cycles of adsorption and elution.
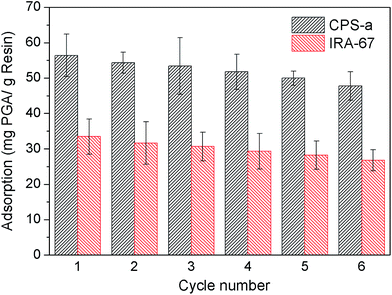 |
| | Fig. 7 Comparison of the reusability of different adsorbents on PGA (PGA concentration: 300 mg L−1, adsorbent amount: 5 g L−1, temperature: 60 °C, reaction time: 60 min). | |
4. Conclusion
A novel adsorbent that consists of PS spheres functionalized with cationic polyelectrolyte (poly-MAC) was prepared and applied as adsorbent for the removal of PGA from aqueous solution. The cationic PS spheres were characterized by FT-IR, 13C NMR, SEM, particle size analyzer, surface area apparatus and elemental analyzer. The results confirmed the modification of poly-MAC on the PS sphere surface. The adsorption of PGA on the cationic PS spheres and on IRA-67 was studied under different experimental conditions. The results clearly demonstrate that graft quantity contributed to the adsorption mechanism through electrostatic interactions between cationic ions and the sites of PGA. The isothermal adsorption behavior of PGA onto cationic PS spheres and IRA-67 followed the Langmuir adsorption model, with a maximum adsorption capacity of 123.07 mg g−1. Kinetic studies of PGA adsorption on cationic PS spheres and IRA-67 revealed that pseudo-first-order function played the most important role in the adsorption process. In the removal of contaminants from whitewater using the adsorption method, it is a promising route to graft functional polymers on the solid matrixes, resulting in high performance.
Note added after first publication
This article replaces the version published on 28th January 2016, which contained an error in the title that was introduced during editorial production.
Notes and references
- M. A. Hubbe, O. J. Rojas and R. A. Venditti, Control of tacky deposits on paper machines – A review, Nord. Pulp Pap. Res. J., 2006, 21, 154–171 CAS.
- M. A. Hubbe, A. Sundberg, P. Mocchiutti, Y. H. Ni and R. Pelton, Dissolved and colloidal substances (DCS) and the charge demand of papermaking process waters and suspensions: a review, BioResources, 2012, 7, 6109–6193 Search PubMed.
- S. Tay, Effect of dissolved and colloidal contaminants in newsprint machine white water on water surface tension and paper physical properties, Tappi J., 2001, 84, 43 CAS.
- D. W. Francis and M. D. Ouchi, Effect of dissolved and colloidal solids on newsprint properties, J. Pulp Pap. Sci., 2001, 27, 289–295 CAS.
- K. Stack, J. H. Zhang, M. Hodgson, T. Lewis and D. Richardson, Effect of increased levels of dissolved and colloidal substances from Pinus radiata on newsprint paper strength, Appita J., 2012, 65, 331–336 CAS.
- L. Wågberg, S. Ondaral and L. Enarsson, Hyperbranched polymers as a fixing agent for dissolved and colloidal substances on fiber and SiO2 surfaces, Ind. Eng. Chem. Res., 2007, 46, 2212–2219 CrossRef.
- L. J. Wang, G. D. Li, Y. Q. Zhang and X. He, Synthesis and evaluation of P(AM-b-DADMAC) as fixative for dissolved and colloidal substances, J. Appl. Polym. Sci., 2013, 130, 4040–4046 CAS.
- V. Donat, T. G. M. Van De Ven and J. Paris, Distribution of dissolved and colloidal substances in the forming and press sections of a paper machine, J. Pulp Pap. Sci., 2003, 29, 294–298 CAS.
- K. Liu, G. L. Zhao, B. H. He, L. H. Chen and L. L. Huang, Immobilization of pectinase and lipase on macroporous resin coated with chitosan for treatment of whitewater from papermaking, Bioresour. Technol., 2012, 123, 616–619 CrossRef CAS PubMed.
- R. N. Wu, B. H. He, G. L. Zhao, L. Y. Qian and X. F. Li, Immobilization of pectinase on oxidized pulp fiber and its application in whitewater treatment, Carbohydr. Polym., 2013, 97, 523–529 CrossRef CAS PubMed.
- J. Nuortila-Jokinen, M. Mänttäri, T. Huuhilo, M. Kallioinen and M. Nyström, Water circuit closure with membrane technology in the pulp and paper industry, Water Sci. Technol., 2004, 50, 217–227 CAS.
- M. Pizzichini, C. Russo and C. D. Di Meo, Purification of pulp and paper wastewater, with membrane technology, for water reuse in a closed loop, Desalination, 2005, 178, 351–359 CrossRef CAS.
- C. Negro, A. Blanco, V. Saarimaa and J. Tijero, Optimization of pitch removal by dissolved air flotation in a eucalyptus kraft mill, Sep. Sci. Technol., 2005, 40, 1129–1143 CrossRef CAS.
- R. Miranda, C. Negro and A. Blanco, Internal treatment of process waters in paper production by dissolved air flotation with newly developed chemicals. 1. laboratory tests, Ind. Eng. Chem. Res., 2009, 48, 2199–2205 CrossRef CAS.
- P. Veverka and K. Jerabek, Mechanism of hypercrosslinking of chloromethylated styrene–divinylbenzene copolymers, React. Funct. Polym., 1999, 41, 21–25 CrossRef CAS.
- N. Bicak, M. Gazi, G. Galli and E. Chiellini, Polystyrene microspheres having epoxy functional dangling chains linked by hydrolytically stable bonds via ATRP, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 6708–6716 CrossRef CAS.
- G. Bayramoglu, B. Karagoz, M. Yilmaz, N. Bicak and M. Y. Arica, Immobilization of catalase via adsorption on poly(styrene-d-glycidylmethacrylate) grafted and tetraethyldiethylenetriamine ligand attached microbeads, Bioresour. Technol., 2011, 102, 3653–3661 CrossRef CAS PubMed.
- S. Willför, A. Sundberg, A. Pranovich and B. Holmbom, Polysaccharides in some industrially important hardwood species, Wood Sci. Technol., 2005, 39, 601–617 CrossRef.
- Z. B. He, Y. H. Ni and E. Zhang, Further understanding on the cationic demand of dissolved substances during peroxide bleaching of a spruce
TMP, J. Wood Chem. Technol., 2004, 24, 153–168 CrossRef CAS.
- G. X. Pan, Relationship between dissolution of fiber materials and development of pulp strength in alkaline peroxide bleaching of mechanical pulp, Holzforschung, 2004, 58, 369–374 CrossRef CAS.
- T. Hannuksela and B. Holmbom, Stabilization of wood-resin emulsions by dissolved galactoglucomannans and galactomannans, J. Pulp Pap. Sci., 2004, 30, 159–164 CAS.
- K. Sundberg, J. Thornton, C. Pettersson, B. Holmbom and R. Ekman, Calcium-induced aggregation of dissolved and colloidal substances in mechanical pulp suspensions, J. Pulp Pap. Sci., 1994, 20, J317–J322 CAS.
- T. M. Garver, T. Y. Xie and K. H. Boegh, Variation of white water composition in a TMP and DIP newsprint paper machine, Tappi J., 1997, 80, 163–173 CAS.
- S. Yeoh, S. Zhang, J. Shi and T. A. G. Langrish, A comparison of different techniques for water-based extraction of pectin from orange peels, Chem. Eng. Commun., 2008, 195, 511–520 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 50
000 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. PGA was purified from orange to a purity higher than 90%. The stock solution was made by dissolving PGA in 0.1 M NaOH over 24 h. This solution was then adjusted to pH 7 with HCl. Crosslinked polystyrene spheres (PS spheres) were kindly supplied by Zhejiang Zhengguang Industrial Co., Ltd., China as shown in Table 1.
000. PGA was purified from orange to a purity higher than 90%. The stock solution was made by dissolving PGA in 0.1 M NaOH over 24 h. This solution was then adjusted to pH 7 with HCl. Crosslinked polystyrene spheres (PS spheres) were kindly supplied by Zhejiang Zhengguang Industrial Co., Ltd., China as shown in Table 1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) and distilled water for 30 min.
100) and distilled water for 30 min.
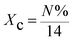
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). The spectrum of cationic PS exhibits some differences such as: (1) the presence of the absorption band at 1726 cm−1, which corresponds to the ester carbonyl group (O
O). The spectrum of cationic PS exhibits some differences such as: (1) the presence of the absorption band at 1726 cm−1, which corresponds to the ester carbonyl group (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O) stretching vibration; (2) appearance of the absorption band at 954 cm−1 belonging to the quaternary ammonium salt from the MAC unit.
C–O) stretching vibration; (2) appearance of the absorption band at 954 cm−1 belonging to the quaternary ammonium salt from the MAC unit.