In vitro human gastro-intestinal enzyme digestibility of globulin isolate from oil palm (Elaeis guineensis var. tenera) kernel meal and the bioactivity of the digest
Abstract
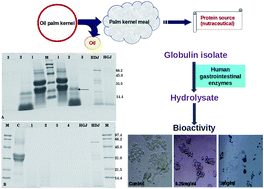
Globulins are the major seed storage proteins (41%) present in oil palm kernel. The study describes the ex vivo digestibility of globulin isolate prepared from oil palm kernel meal. The bioactivity and nutritional value of the ex vivo digest of globulin isolate was also investigated. Globulin isolate (85% protein content) was prepared by a salt extraction method and its digestibility was studied using human gastroduodenal juices (HGDJ). By SDS-PAGE analysis it was evident that the globulin isolate was completely digested by HGDJ. Easy digestibility of a dietary protein is essential from the nutritional point of view. In the present ex vivo study, the digestion profile and degree of hydrolysis of globulin isolate were observed to be comparable with that of casein. RADVFNPR and KLPLVERIP were the two peptides identified by MS/MS analysis in the final hydrolysate. By pepsinolysis, it was evident that the novel globulin protein preparation was devoid of pepsin resistant proteins that are otherwise considered to have allergenic potential. The ex vivo hydrolysate exhibited potent ACE-inhibitory activity (IC50 50 μg ml−1) and anticancer activity against human colon epithelial cancer HT-29 cells and hepatocarcinoma HepG2 cells. Easy digestibility, bioactivity, the presence of nutritionally important free essential amino acids and non-protein amino acids (γ-aminobutyric acid and citrulline) in the gastrointestinal digest suggests that the globulin isolate prepared from palm kernel meal can be used as a nutraceutical protein for food applications.


 Please wait while we load your content...
Please wait while we load your content...