Enzyme-mediated in situ formation of pH-sensitive nanogels for proteins delivery†
Zhipeng Zenga,
Yingqi Shea,
Zhiping Peng*a,
Junchao Weib and
Xiaohui Hea
aSchool of Materials Science and Engineering, Nanchang University, Nanchang 330031, China. E-mail: zzpeng@ncu.edu.cn
bCollege of Chemistry, Nanchang University, Nanchang 330031, China
First published on 14th January 2016
Abstract
Novel pH-sensitive nanogels based on poly(ethylene glycol)-b-poly(L-glutamate-g-tyramine) (PEG-b-P(LGA-g-Tyr)) copolymer were developed for efficiently delivering and releasing proteins into HeLa cells. The core–shell nanogels were in situ fabricated through the enzyme-catalyzed oxidative coupling of tyramine moieties in the core of the self-assembled PEG-b-P(LGA-g-Tyr) micelles in the presence of horseradish peroxidase (HRP) and hydrogen peroxide (H2O2). The stable nanogels have spherical morphology with an average diameter of about 125 nm under physiological condition. The pH-dependent size shrink of the nanogels was observed by dynamic light scattering (DLS). Fluorescein isothiocyanate conjugate bovine serum albumin (FITC-BSA) was in situ incorporated into the nanogels with an entrapment efficiency of 69.9% during the enzyme-catalyzed crosslinking reaction. In vitro protein release profiles at pH 7.4 and pH 6.8 showed a burst effect followed by a continuous release phase. The FITC-BSA loaded nanogels exhibited pH-sensitive protein release. A significantly fast FITC-BSA release was observed at endosomal pH than at physiological pH. The cumulative release of FITC-BSA from the nanogels at pH 7.4 and pH 6.8 were 24.2% and 40.3%, respectively. Cell Counting Kit-8 (CCK-8) assay showed that these nanogels were non-toxic up to a concentration of 2.0 mg mL−1. Confocal laser scanning microscopy (CLSM) studies revealed that FITC-BSA loaded nanogels efficiently delivered and released proteins into HeLa cells. We are convinced that these enzymatically crosslinked nanogels with excellent biocompatibility and pH-responsibility have a promising potential for protein delivery system.
Introduction
Protein therapeutics are becoming available for the effective treatments of various diseases due to their high specificity, superior therapeutic activity, and low cytotoxicity.1 Because of the fragile nature of proteins,2 the clinical success of protein therapy depends on the development of safe and efficient protein delivery systems to improve the bioavailability and protect the active therapeutic from premature degradation.3 In the past decades, a series of nanocarriers have been developed for improving the stability and bioavailability of therapeutic proteins.4–6Nanogels are nanometer-sized hydrogel particles formed by physically or chemically crosslinked polymer networks.7,8 Nanogels are very promising as one of the mostly ideal protein delivery carriers because of their distinct advantages, including high stability, relatively uncomplicated fabrication, high protein loading capacity, and long circulation time in plasma.9 Stimuli-responsive nanogels which respond to external stimuli (such as temperature, pH, light, etc.) have attracted considerable attention.4,10,11 It is well known that the pH value in tumor tissues is relatively acidic (pH 5.5–7.0) as compared with normal tissues.12,13 This acidic condition at tumor sites has been supposed as an ideal stimulus for the selective release of anticancer drugs into tumors to achieve tumor targeted drug delivery. Many pH-sensitive polymeric nanocarriers have been developed for tumor targeted and intracellular drug release.13–17 In particular, pH-sensitive nanogels that release loaded proteins cargo in response to endosomal pH have received increasing interesting.4,18–22 Akiyoshi et al. synthesized pH-sensitive cholesteryl-modified pullulan (CHP) nanogels as protein delivery vehicles.18 The CHP nanogels were modified by cell specific peptide (Arg-Gly-Asp; RGD) and used as targeted protein delivery. The BSA/RGD-modified nanogels were efficiently internalized into cells through integrin-mediated endocytosis.20 Baik et al. fabricated polysaccharide nanogels consisting of BSA and 3-diethylaminopropyl (DEAP) groups grafted glycol chitosan (GCS-g-DEAP). The GCS-g-DEAP/BSA nanogels exhibited an improved cellular uptake of BSA, an enhanced blood circulation and a high accumulation of BSA in the tumor site.21
Generally, the most common synthetic approaches for the preparation of nanogels can be grouped into two different categories: physical crosslinking and covalent crosslinking method.8,23 The physically crosslinked nanogels are formed by the self-assembly of interactive polymers via non-covalent interactions, such as hydrophobic interactions, electrostatic interactions and hydrogen bonding. The formation of these nanogels is conducted under mild conditions, and the nanogels are extremely useful for the encapsulation of protein. However, the stability of physically self-assembled nanogels is still a major challenge in delivering drugs to tissues and cells. The covalent crosslinking technique provides a popular choice for synthesis of a variety of functional nanogels for drug delivery. These nanogels are fabricated by covalent coupling of the reactive functional groups in monomers or preformed polymers to form the gel networks. The covalently crosslinked nanogels exhibit excellent structural and colloidal stability.24 Various covalent crosslinking strategies have been utilized to prepare nanogels. They include radical crosslinking copolymerization of vinyl monomers, click chemistry, Schiff-base reaction, thiol-disulfide exchange reaction, photo-induced crosslinking and enzyme-catalyzed crosslinking.7,25 However, many covalent crosslinking reactions are conducted in the presence of surfactant, cytotoxic crosslinking agents or harsh experimental conditions, which may adversely affect the biological actively of the encapsulated protein therapeutics.
Among the various covalent crosslinking methods, enzyme-catalyzed crosslinking reactions with high selectivity and specificity are raising significant interest. The preformed polymers can be crosslinked by enzyme-mediated crosslinking under physiologic conditions without unwanted side reactions and cytotoxicity.26 Recently, in situ formed hydrogels by enzyme-mediated crosslinking using HRP as an oxidoreductase and H2O2 as the oxidizing agent are rapidly gaining mindshare because of their excellent biocompatibility, fast gelation process, tunable mechanical properties and low immunogenicity.27–30 Although H2O2 has the potential to create a cytotoxic environment for cells or to deactivate proteins, the low concentration and rapidly the consumption rate of H2O2 during the enzyme-catalyzed crosslinking reactions allows therapeutic proteins, growth factors and cells to be incorporated without compromising their biological activities.31–35 As their high convenience and biocompatibility, these injected hydrogels formed by HRP-catalyzed crosslinking were used as carriers for protein and cell delivery. For instance, release of protein (α-amylase and lysozyme) in a diffusion controlled manner was accomplished from enzymatically crosslinked hyaluronic acid-tyramine (HA-Tyr) hydrogels. More than 95% of released α-amylase retained its bioactivity which suggested that the HRP-catalyzed crosslinking reactions did not cause denaturation of the proteins.33 Interferon-2α (IFN) was incorporated in HA-Tyr hydrogel to develop the injectable therapeutically effective drug carrier for liver cancer therapy.35 Recently, our group developed enzymatically crosslinked poly(γ-glutamic acid)-tyramine (PGA-Tyr) hydrogels as a delivery system for controlled release of BSA. The diffusion behaviours of BSA in the PGA-Tyr hydrogels can be manipulated by controlling the crosslink density and mesh size of hydrogels.36 Although enzymatically crosslinked hydrogels for drug delivery have been widely investigated, nanogels with desirable sizes (10–200 nm) and long circulation time are more suitable for tumor targeted and intracellular drug release compared with the bulk hydrogels.7,8 It is reasonable to assume that enzyme-crosslinking reactions provides a novel, green and fast method for the preparation of bioactive molecule loaded nanogels. However, the enzymatically crosslinked nanogels have not been widely investigated.37–40 Therefore, there is a growing interest in developing enzyme-mediated in situ formation of nanogels for tumor targeted and intracellular drug delivery. For example, Groll et al. reported redox-sensitive disulfide-crosslinked nanogels by HRP-mediated crosslinking. β-Galactosidase as a model protein was encapsulated into the nanogels and released without loss of vitality under the cytosol-like reductive conditions.37 Recently, Haag's group developed a new nanogel preparation method for protein encapsulation by HRP-catalyzed oxidative crosslinking on phenolic derivatized dendritic polyglycerol (dPG) in the presence of H2O2 in an inverse miniemulsion. The dPG nanogels provide efficient scaffolds for active protein encapsulation.38
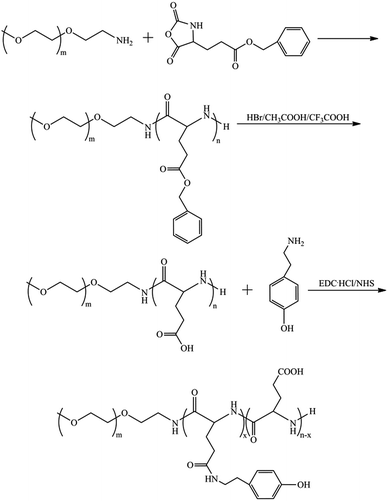
In this paper, we reported in situ forming core–shell nanogels based on poly(ethylene glycol)-b-poly(L-glutamate-g-tyramine) (PEG-b-P(LGA-g-Tyr)) under physiological condition using an enzymatic oxidative coupling reaction in the presence of HRP and H2O2. Poly(L-glutamic acid) (PLGA) is an anionic polymer with favourable physicochemical properties including modifiable carboxyl side group, excellent biocompatibility and pH-responsive property (pKa ∼ 4.5).30,41 Recently, Ren et al. developed enzymatically crosslinked poly(L-glutamic acid) grafted with tyramine and poly(ethylene glycol) (PLG-g-TA/PEG) hydrogels using HRP and H2O2.30 Herein, PEG-b-P(LGA-g-Tyr) core–shell nanogels were prepared via three steps reaction (Scheme 1). Initially, PEG-b-PLGA copolymer was synthesized through ring-opening polymerization (ROP) of γ-benzyl-L-glutamate-N-carboxy anhydride (BLG-NCA) and deprotection of benzyl group. PEG-b-P(LGA-g-Tyr) was then synthesized by grafting tyramine to PLGA block. Then, the pendant tyramine groups in PEG-b-P(LGA-g-Tyr) were crosslinked by HRP-catalyzed oxidation reaction in very dilute H2O2 solution to form core-crosslinked nanogels. The hydrophilic PEG shell endows PEG-b-P(LGA-g-Tyr) nanogels high colloidal stability and long circulation time in blood which are the major challenge of nanogels for intravenous or intracellular drug delivery. The diblock copolymer and pH-sensitive nanogels were characterized by 1H-NMR, UV-vis spectroscopy, fluorescence spectroscopy, dynamic light scattering (DLS), scanning electron microscope (SEM) and transmission electron microscopy (TEM). FITC-BSA as a model protein was efficiently in situ loaded in nanogels under mild conditions and released in a pH-controlled manner. The potential applicant of these nanogels as intracellular protein delivery nanocarriers was evaluated using CLSM for cellular uptake and cell viability measurements.
Experimental
Materials
α-Methoxy-ω-amino poly(ethylene glycol) (CH3O-PEG-NH2, Mw = 5000) was purchased from Shanghai Yare Biotech. Inc. Trifluoroacetic acid, γ-benzyl-L-glutamate (BLG) and 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC·HCl) were obtained from Energy Chemical. 45% w/w HBr/CH3COOH was provided by Alfa Aesar. Tyramine hydrochloride, Bull Serum Albumin (BSA), horseradish peroxidase (HRP) and fluorescein isothiocyanate (FITC) were obtained from Sigma-Aldrich. Dimethylformamide (DMF) and hydrogen peroxide (H2O2) were supplied from Sinopharm Chemical Reagent Co. Ltd. γ-Benzyl-L-glutamate-N-carboxy anhydride (BLG-NCA) was synthesized according to the procedure reported in the literature.42Characterization
The 1H-NMR of PEG-b-PBLG, PEG-b-PLGA and PEG-b-P(LGA-g-Tyr) were performed on a Bruker ARX 600 NMR spectrometer at ambient temperature. The molecular weight and polydispersity of PEG-b-PBLG copolymer were determined by gel permeation chromatography (GPC, Waters 2410) in DMF at room temperature. The fluorescence spectra of FITC-BSA were determined by a fluorescence spectrophotometer (Hitachi F-7000). For fluorescence emission spectra, the excitation wavelength was 490 nm, and the excitation bandwidth was 2.5 nm. The spectra were recorded from 500 to 800 nm with a scanning rate 1200 nm min−1. A scanning electron microscope (SEM, FEI Quanta 200SEM) was used to observe the surface morphology and diameter of nanogels at an accelerating voltage of 20 kV. The sample was sputter-coated with aurum for analysis. The diameter of the nanogels was measured from the SEM micrographs. In addition, morphology of the nanogels was analyzed using transmission electron microscopy (TEM, JEOL-2100F) at 150 kV. Samples for TEM were prepared by directly depositing the nanogels solution onto carbon-coated copper grids. The size distribution of the PEG-b-P(LGA-g-Tyr) micelles and nanogels was determined using dynamic light scattering (DLS). Measurements were carried out at 1.0 g L−1 copolymers or nanogels concentration in PBS solution (pH 7.4, 6.8 and 5.4) using a PAS (NANO2590) from Malvern Instruments. The size stability of nanogels was evaluated by DLS during long-term storage in buffer solution (pH 7.4). The ultraviolet-visible (UV) spectra of the samples were recorded on a PerkinElmer Lambda 750 spectrophotometer.Synthesis of poly(ethylene glycol)-b-poly(L-glutamate) (PEG-b-PLGA)
Poly(ethylene glycol)-b-poly(γ-benzyl-L-glutamate) copolymer was synthesized by ring-opening polymerization of BLG-NCA using PEG-NH2 as macroinitiator.43 Briefly, PEG-NH2 (0.25 g, 0.05 mmol) and BLG-NCA (0.9 g, 3.4 mmol) were dissolved in anhydrous DMF, and the solution was stirred for 48 h at room temperature under a nitrogen atmosphere. The reaction mixture was poured into excessive anhydrous diethyl ether under stirring to obtain a white solid. The obtained product was further purified with ethanol for several times, and drying overnight under vacuum (yield 81%). PEG-b-PLGA was prepared by debenzylation of PEG-b-PBLG using HBr.30 PEG-b-PBLG (0.5 g) was dissolved in 5 mL of trifluoroacetic acid in a flask at 0 °C and then 1.5 mL of 33 wt% HBr/CH3COOH was added to the solution. The solution was slowly stirred at 30 °C for 1 h and then the final product was precipitated into excessive anhydrous diethyl ether. The precipitate was collected and dialyzed against distilled water for 48 hours. After dialysis, the solution was freeze-dried to give PEG-b-PLGA (yield 71%).Synthesis of poly(ethylene glycol)-b-poly(L-glutamate-g-tyramine) (PEG-b-P(LGA-g-Tyr))
PEG-b-P(LGA-g-Tyr) was prepared as described previously with some modifications.28 PEG-b-PLGA (0.3 g) was dissolved in 50 mL of distilled water. EDC·HCl (0.382 g, 2 mmol) and NHS (0.23 g, 2 mmol) were added to this solution and stirred for 30 min to activate the carboxyl groups of PEG-b-PLGA. Then, tyramine hydrochloride (0.34 g, 2 mmol) was added and the pH of the reaction mixture was maintained at 5.8 with 1 M NaOH or 1 M HCl. The reaction mixture was stirred overnight at room temperature, and then was dialyzed (molecular weight cut off = 3500) against distilled water for 3 day. The purified solution was lyophilized to obtain PEG-b-P(LGA-g-Tyr) (yield 77%).Preparation of FITC-BSA
As a fluorescent marker, FITC was labeled on BSA.44 Briefly, BSA (0.50 g) was dissolved in 100 mL of deionized water containing 10 mg of FITC. The solution was stirred for 4 h and dialyzed against water until no fluorescence was detected from the outer water phase. After dialysis, the solution was freeze-dried to obtain FITC-BSA. The product was stored at 4 °C prior to use.Measurement of critical aggregation concentration (CAC)
The CAC of the PEG-b-P(LGA-g-Tyr) and nanogels was determined via fluorescence spectroscopy using pyrene as the fluorescence probe.45 The PEG-b-P(LGA-g-Tyr) and nanogels solution was prepared with polymer concentration from 1.0 × 10−4 to 1.0 g L−1. The pyrene final concentration in each sample solution was fixed at 6.0 × 10−7 mol L−1. The emission wavelength was 394 nm, and the slit width was 2.5 nm.In situ formation of core–shell nanogels
Core–shell nanogels based on PEG-b-P(LGA-g-Tyr) copolymer were prepared by crosslinking of tyamine units using HRP-catalyzed oxidation reaction. PEG-b-P(LGA-g-Tyr) copolymer (20 mg) was dissolved in 2 mL of DMSO and dialyzed against phosphate buffered saline (PBS, pH 7.4, 10 mM). After 3 days, the dialysate was collected and their volumes adjusted to 20 mL (final concentration 1 mg mL−1). Then HRP (50 μL, 1.25 unit per mL) and H2O2 (50 μL, 50 mmol L−1) were added to the solution. The mixture solution was stirred for 2 h at 37 °C to enzymatically form nanogels.Encapsulation and triggered release of FITC-BSA
FITC-BSA (5 mg) was added to 10 mL of PEG-b-P(LGA-g-Tyr) copolymer solution (1 mg mL−1) in PBS (pH 7.4, 10 mM), then HRP (50 μL, 1.25 unit per mL) and H2O2 (50 μL, 50 mmol L−1) were added to enzymatically form nanogels. The FITC-BSA loaded nanogels were captured by centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and washed three times using deionized water to remove the free FITC-BSA completely. The drug-loaded nanogels were collected by freeze-drying. All supernatant solutions were collected, and the FITC-BSA concentration in the supernatant solution was analyzed using a fluorescence spectroscopy at a wavelength of maximum absorbance (520 nm). The protein loading efficiency PLE = (W0 − W1)/W0 and protein loading contents PLC = (W0 − W1)/Wnano in these nanogels were calculated. Here W0, W1 and Wnano are the initial weight of FITC-BSA in the loading solution, residual weight of FITC-BSA in the supernatant solution and the weight of nanogels, respectively.
000 rpm and washed three times using deionized water to remove the free FITC-BSA completely. The drug-loaded nanogels were collected by freeze-drying. All supernatant solutions were collected, and the FITC-BSA concentration in the supernatant solution was analyzed using a fluorescence spectroscopy at a wavelength of maximum absorbance (520 nm). The protein loading efficiency PLE = (W0 − W1)/W0 and protein loading contents PLC = (W0 − W1)/Wnano in these nanogels were calculated. Here W0, W1 and Wnano are the initial weight of FITC-BSA in the loading solution, residual weight of FITC-BSA in the supernatant solution and the weight of nanogels, respectively.
In vitro protein release from pH-sensitive PEG-b-P(LGA-g-Tyr) nanogels was investigated using a dialysis method (molecular weight cut off = 100 kDa) at 37 °C in PBS with different pH (pH 6.8 and pH 7.4). At desired time intervals, 1 mL of release media was taken out and replenished with an equal volume of corresponding fresh media. The concentration of released protein was determined by fluorescence measurements. The release experiments were performed in triplicates and average values were reported.
CCK8 assay
The cytotoxicity of blank nanogels and FITC-BSA-loaded nanogels was evaluated by Cell Counting Kit-8 (CCK-8) assay with HeLa cells. The cells were collected and diluted to a cell density of 1 × 105/mL in complete medium, and then seeded into a 96-well plate (100 μL per well). After being cultured for 24 h, blank nanogels and FITC-BSA loaded nanogels with different concentrations were added and the cells were incubated for another 24 h (37 °C, 5% CO2). After designated incubation time, 10 μL of CCK8 solution (5.0 mg mL−1) was added to every plate. After culturing 1 h in incubators, the optical density (OD) at 450 nm was investigated by ELIASA. The viability of cells of untreated blanks (no nonogels, no FITC-BSA) was normalized to 100%. Five experimental sets were carried out, and each set was performed in triplicate.Confocal microscopy observation of HeLa cells incubated with FITC-BSA loaded nanogels
HeLa cells were placed on microscope slides in a 24-well plate (5 × 104 cells per well) using DMEM medium containing 10% FBS. The cells were incubated with predetermined amounts of FITC-BSA loaded nanogels or free FITC-BSA at 37 °C under 5% CO2 atmosphere. After 4 h incubation, the culture medium was removed and the cells on microscope plates were washed three times with PBS. The cells were fixed with 4% paraformaldehyde and the cell nuclei were stained with Hoechst 33342. Fluorescence images of cells were observed with a Nikon Digital Eclipse C1si Confocal Laser Scanning Microscope (Nikon, CLSM).Results and discussion
Synthesis of PEG-b-P(LGA-g-Tyr) copolymers
PEG-b-P(LGA-g-Tyr) block copolymer was synthesized by ring-opening polymerization of BLG-NCA, deprotection of benzyl group and subsequent amidation reaction with amino of tyramine (Scheme 1). PEG-b-PLGA diblock copolymer was used to fabricate nanogels due to its biodegradability and pH-responsive property. The structures of the PEG-b-PBLG, PEG-b-PLGA and PEG-b-P(LGA-g-Tyr) copolymers were confirmed by the 1H NMR spectra. The typical 1H NMR spectrum of PEG-b-PBLG in CDCl3 was shown in Fig. 1a. The peaks at δ ∼ 7.27 ppm (5H, Ar–H) (f), δ ∼ 5.05 ppm (2H, Ar–CH2–) (e), δ ∼ 4.0 ppm (1H, –NH–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –CO–) (b), δ ∼ 2.28 and 2.13 ppm (2H, –CH2–CH2–CO–) (d and c) ascribe to the protons of PBLG segments.46 The polymerization degree of the PBLG block (DPBLG) was estimated by the NMR peak areas at 5.05 ppm (e) of PBLG block and at 3.65 ppm (a) of the PEG methylene protons. The DPBLG was calculated to be 57, and the block copolymer molecular weight was estimated to be 17
–CO–) (b), δ ∼ 2.28 and 2.13 ppm (2H, –CH2–CH2–CO–) (d and c) ascribe to the protons of PBLG segments.46 The polymerization degree of the PBLG block (DPBLG) was estimated by the NMR peak areas at 5.05 ppm (e) of PBLG block and at 3.65 ppm (a) of the PEG methylene protons. The DPBLG was calculated to be 57, and the block copolymer molecular weight was estimated to be 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400. The number average molecular (Mn) of PEG-b-PBLG copolymer was determined to be 1.6 × 104 with polydispersity index (PDI) of 1.23 by GPC. As shown in Fig. 1b, typical signals of both PEG and PLGA units were detected in D2O. The peak at δ ∼ 3.73 ppm (4H, –CH2–CH2–O–) (a) is attributed to the protons of the PEG unit. The peaks at δ ∼ 4.35 ppm (1H, –NH–C
400. The number average molecular (Mn) of PEG-b-PBLG copolymer was determined to be 1.6 × 104 with polydispersity index (PDI) of 1.23 by GPC. As shown in Fig. 1b, typical signals of both PEG and PLGA units were detected in D2O. The peak at δ ∼ 3.73 ppm (4H, –CH2–CH2–O–) (a) is attributed to the protons of the PEG unit. The peaks at δ ∼ 4.35 ppm (1H, –NH–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –CO–) (b), δ ∼ 2.28, and δ ∼ 1.93 ppm (2H, –CH2–CH2–CO–) (d and c) are characteristic of proton peaks of PLGA.47 After grafting with tyramine, new peaks at δ ∼ 6.98 and ∼6.68 (g and h) assigned to tyramine units were observed in Fig. 1c. It demonstrated that PEG-b-P(LGA-g-Tyr) conjugates were successfully synthesized.30 The grafted number of tyramine groups per PEG-b-P(LGA-g-Tyr) macromolecular chain was estimated to be 17 by the peak intensities of the tyramine proton signal (6.98–6.68 ppm) (g and h) and ethylene proton signal (about 3.6 ppm) (a) of PEG in the 1H NMR spectrum.
–CO–) (b), δ ∼ 2.28, and δ ∼ 1.93 ppm (2H, –CH2–CH2–CO–) (d and c) are characteristic of proton peaks of PLGA.47 After grafting with tyramine, new peaks at δ ∼ 6.98 and ∼6.68 (g and h) assigned to tyramine units were observed in Fig. 1c. It demonstrated that PEG-b-P(LGA-g-Tyr) conjugates were successfully synthesized.30 The grafted number of tyramine groups per PEG-b-P(LGA-g-Tyr) macromolecular chain was estimated to be 17 by the peak intensities of the tyramine proton signal (6.98–6.68 ppm) (g and h) and ethylene proton signal (about 3.6 ppm) (a) of PEG in the 1H NMR spectrum.
 | ||
| Fig. 1 1H NMR of spectra of (a) PEG-b-PBLG in CDCl3, (b) PEG-b-PLGA in D2O, (c) PEG-b-P(LGA-g-Tyr) in DMSO-d6 and (d) in D2O. | ||
Fig. 2 contains the UV-vis absorbance spectra of PEG-b-PLGA and PEG-b-P(LGA-g-Tyr) conjugates aqueous solutions of 0.1 wt%. The characteristic absorbance peak of the tyramine48 at 275 nm in PEG-b-P(LGA-g-Tyr) spectrum was found compared with PEG-b-PLGA. It confirmed the successful chemical bonding of the tyramine moieties on the side chain of PEG-b-PLGA copolymer. The content of introduced tyramine groups was calculated from a calibration curve which was obtained by measuring the absorbance of known percentages of tyramine hydrochloride in distilled water. The tyramine concentration was estimated to be 2.1 wt% that corresponded to incorporated tyramine groups in per PEG-b-P(LGA-g-Tyr) macromolecular chain were about 20. It is consistent with the NMR result.
Formation of pH-sensitive core–shell nanogels
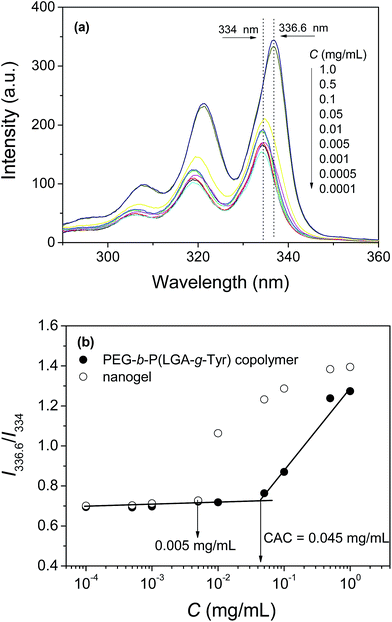
The amphiphilic PEG-b-P(LGA-g-Tyr) copolymer grafted with hydrophobic tyramine groups can self-assemble into micelles in aqueous solution. The self-assembly behaviour of PEG-b-P(LGA-g-Tyr) in aqueous solution was monitored via a pyrene fluorescence technique. The excitation spectra of pyrene probe in PEG-b-P(LGA-g-Tyr) aqueous solution at various concentrations were measured as presented in Fig. 3a.With increasing copolymer concentration, the (0, 0) band of pyrene probe shifted from 334 nm to 336.6 nm which indicated that pyrene transferred from water phase to the hydrophobic domain.45 The change in the intensity ratio (I336.6/I334) of pyrene in PEG-b-P(LGA-g-Tyr) solution was used to determine the CAC. Fig. 3b depicts the ratio of I336.6/I334 of pyrene probe as a function of PEG-b-P(LGA-g-Tyr) and nanogels concentration. The CAC was determined as 0.045 mg mL−1 from the intersection point of the tangents to the curve. Above CAC, the PEG-b-P(LGA-g-Tyr) copolymers tend to form micelles in aqueous solution which is composed of PEG shell and P(LGA-g-Tyr) core. The grafted tyramine moieties are assembled in the core of the PEG-b-P(LGA-g-Tyr) micelles which provides the site for the following HRP-catalyzed oxidation reaction. The excitation spectra of pyrene probe in nanogels aqueous solution at various concentrations are shown in Fig. S1.† The characteristic spectrum of pyrene in water was observed and the ratios of I336.6/I334 remain the same when the concentrations of nanogels below 0.005 mg mL−1, which indicate that most of pyrene stay in water in very dilute nanogels solution. The (0, 0) band of pyrene probe shifted suddenly from 334 nm to 336.6 nm in the range of nanogels concentration of 0.005 to 0.01 mg mL−1. These results suggest that the pyrene probe transfer into the hydrophobic core of the nanogels from water in the range of nanogels concentration of 0.005 to 0.01 mg mL−1. With increasing the concentration of nanogels from 0.005 to 1 mg mL−1, more pryene could transfer into the core of the nanogels which results in a dramatic increase of the ratio of I336.6/I334. It seems that the CAC of the core-crosslinked nanogels should be 0.005 mg mL−1. Actually, the CAC determination does not have any physical meaning for crosslinked nanogels.49 The CAC is governed by the equilibrium between unimer and micelle. For core-crosslinked nanogels, the micelle structure is irreversible fixed by covalent crosslinking. The change of the ratio of I336.6/I334 in the nanogels system just shows that the partition of pyrene between water and the hydrophobic core of nanogels. Therefore, the concept of CAC is not applicable to the crosslinked nanogels.49,50 However, the extremely low “CAC” in the nanogels system may be used to indicate the excellent stability of nanogels.51
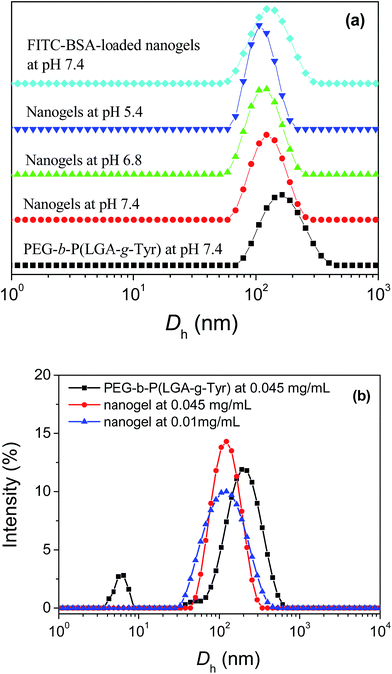
As we known, the self-assembly process as aggregation results in a decreased mobility of the core-forming block and a resultant suppression of NMR signal intensities.52 The structure of resulting core–shell micelles was further characterized by 1H NMR analyses in D2O in Fig. 1d. The signals corresponding to the PEG shell (3.6 ppm) were obviously observed, while the signals from the protons of tyramine moieties (6.98–6.68 ppm) (g and h) in the micelles core were almost completely suppressed in the 1H NMR spectrum. The peak areas ratio between the protons of tyramine at 6.98–6.68 ppm and ethylene protons at about 3.6 ppm in Fig. 1d markedly decreased compared with that in Fig. 1c. These results were attributed to the reduced mobility of the P(LGA-g-Tyr) segments in the inner core. The self-assembly behavior of PEG-b-P(LGA-g-Tyr) was further verified by DLS. As shown in Fig. 4a, there is a broad hydrodynamic diameter (Dh) distribution peaks (particle dispersion index, PDI = 0.243) at Dh ≈ 159 nm in PEG-b-P(LGA-g-Tyr) PBS solution (at pH 7.4) which corresponds to the micelle.
The core–shell nanogels were fabricated through the enzyme-catalyzed crosslinking of the tyramine moieties in the P(LGA-g-Tyr) core of self-assembled micelles using HRP and H2O2 (Scheme 2). HRP is frequently used as a catalyst for oxidative coupling of phenol derivatives under mild reaction conditions. It is well known that phenols crosslink through either a more common C–C linkage between the ortho-carbons of the aromatic ring or a C–O linkage between the ortho-carbon and the phenolic oxygen.32
The successful formation of the core crosslinked nanogels was investigated by DLS analysis, SEM and TEM. The hydrodynamic diameter of formed nanogels slightly decreased to about 125 nm (PDI = 0.215) at pH 7.4 (Fig. 4a). The smaller size of nanogels compared with that of copolymer micelles suggested a more compact structure after enzymatic oxidation reaction of tyramine moieties.48 The colloidal stability of micelles and nanogels was evaluated by the size stability in Fig. 4b. The nanogels were found to be highly stable after diluting to 0.01 g L−1, showing negligible changes in their sizes. However, there are two Dh distribution peaks in PEG-b-P(LGA-g-Tyr) solution at CAC which correspond to the unimer (Dh ≈ 5.8 nm) and micelle (Dh ≈ 208 nm). As we known, the loose aggregates formed by the collapsed blocks maintain equilibrium with the unimer at CAC. The core of micelles formed at CAC contains a significant amount of solvent which results in that the sizes of the micelles formed at CAC are larger than those formed at higher concentration.53 No aggregates can be determined by DSL in the dilute PEG-b-P(LGA-g-Tyr) solution (0.01 g L−1) because the count rate was too low. These results show that the PEG-b-P(LGA-g-Tyr) micelles dissociate into free copolymer chains below CAC whereas nanogels maintain their core–shell structures even far below the CAC of micelles. The colloidal stability is crucial for intracellular drug delivery. The micelles may disintegrate into unimers and premature release of the loaded drug at unexpected locations due to the body fluids dilution in vivo circulation. The size stability of the PEG-b-P(LGA-g-Tyr) micelles and nanogels during long-term storage in buffer solution (pH 7.4) at concentration of 1 g L−1 was also investigated by DLS (Fig. S2†). The aggregation of micelles occurred and leaded to precipitation in the dispersed suspension of PEG-b-P(LGA-g-Tyr) micelles during storage for about 10 days. The nanogels maintained their initial colloidal suspension state in PBS (pH 7.4) without any precipitation for at least 20 days. Fig. S3† shows the change in the size of the nanogels over time. The size of the nanogels is almost identical to the initial size during storage. The excellent stability of these nanogels provides the feasibility of their long circulation time.
SEM and TEM images of the nanogels are given in Fig. 5a–c. The SEM and TEM micrographs showed that the core crosslinked nanogels had regular spherical morphology with average diameter of around 100 nm. The size of these nanogels examined by SEM and TEM is slightly smaller than that measured by DLS. This discrepancy should be attributed to the dehydration of the nanogels in the sample preparation process for SEM and TEM.
 | ||
| Fig. 5 SEM images of nanogels (a) the scale bar represents 3 μm and (b) the scale bar represents 500 nm, (c) TEM image of nanogels, the scale bar represents 100 nm. | ||
The pH-dependence properties of formed nanogels were also determined by DLS in PBS solution at pH 7.4, 6.8 and 5.4 (Fig. 4a). The Dh of nanogels decreased from 125 nm to 113 nm (PDI = 0.215) at pH 6.8 and 109 nm (PDI = 0.193) at pH 5.4. The pH induced protonation of PLGA segments resulted in the enhanced hydrophobic interaction which leaded to more compact core and smaller size of the nanogels. These results confirmed that the pH-sensitive core crosslinked nanogels were successfully prepared via the enzyme-catalyzed crosslinking of the self-assembled PEG-b-P(LGA-g-Tyr) micelles. The appropriate size of nanogels empowers these nanogels to accumulate in tumor tissues through enhanced permeability and retention (EPR) effect.54
Loading and pH-sensitive triggered release of FITC-BSA
FITC-BSA as a model protein was in situ encapsulated in PEG-b-P(LGA-g-Tyr) nanogels during the HRP-catalyzed crosslinking reaction to investigate the feasibility of using nanogels as protein carriers. The characteristic absorption at 520 nm of FITC-BSA (Fig. S4†) was used to determine the PLC and PLE of the FITC-BSA loaded nanogels in PBS (pH 7.4). The PLC and PLE were estimated to be 36.8% and 69.9% according to the calculation formula, respectively. The high protein loading capability of nanogels might be attributed to two reasons. On the one hand, most of FITC-BSA was incorporated into the core of nanogels by the electrostatic interaction between the negatively charged carboxyl groups in P(LGA-g-Tyr) chains and the patches of positive charge of BSA. The strong electrostatic interaction is caused by the counterion release forces.55 The patches of positive charge on the surface of BSA become multivalent counterions of the charged carboxyl groups in PLGA chains. As we known, the isoelectric point (pI) of BSA is about 4.5 and the pKa of PLGA is also about 4.5. The BSA and the PLGA block carry the same net charge at pH 7.4 in our loading experiment. The adsorption of negatively charged BSA onto negatively charged PLGA chains may appear surprising. This phenomenon is referred to as “on the wrong side” to indicate the interaction of a polyanion with a protein at a pH above its isoelectric point.56 Ballauff's group has been demonstrated in detail the spherical poly(acrylic acid) (PAA) brushes strongly adsorption of BSA on the wrong side of the isoelectric point.55,57–59 This unexpected finding can be explained by the presence of positive patches on the surface of the protein which persists far above the isoelectric point.60 On the other hand, small amount of FITC-BSA was embedded in the PEG shell via hydrogen bonding between the chain backbone of PEG and BSA.61 FITC-BSA was physically absorbed on the shell of nanogels and entrapped within the core of nanogels, the size of nanogels was found to increase about 10 nm compared with the unloaded nanogels in Fig. 4a.As shown in Fig. S5,† FITC appears in its various forms (cation, neutral molecule, monoanion and dianion, pKa1 = 2.1, pKa2 = 4.3, and pKa3 = 6.4, respectively) at different pH, which lead to the fluorescence properties of FITC strongly dependent upon pH.62 The dianion form predominates at pH values above 6.4. It has the most intense fluorescence with a quantum yield of 0.93 and is widely used for determining quantificationally FITC-labelled protein.10 In the pH range of 4.3 to 6.4, FITC exists in the monoanion form with a fluorescence quantum yield of 0.37 which is unsuitable for quantifying FITC-labelled protein. The fluorescence spectra of FITC-BSA loaded nanogels were measured at different pH (Fig. 6). The fluorescence spectra of FITC-BSA loaded nanogels at pH 7.4 and 6.8 are selfsame. The maximum absorption at 520 nm which has been attributed to the dianion species was used to evaluate the release behaviours of loaded FITC-BSA from nanogels. The fluorescence intensity decreased markedly with the decreasing pH which in accordance with data in literature.63 At acidic solutions (pH 4.0), the fluorescence spectrum of FITC-BSA loaded nanogels showed a 5 nm blue-shift which indicates that encapsulated FITC-BSA in acid condition is located in a more-hydrogen-donating and hydrophobic environment.64 The protonation of carboxyl groups on PLGA segments in acid condition leads to the more hydrophobic micro-environment. This result was consistent with DLS consequence and confirmed that most FITC-BSA was loaded into the core of nanogels.
The in vitro release behaviours of loaded FITC-BSA from nanogels were evaluated at 37 °C under simulated physiological conditions (pH 7.4) and cancer tissue (pH 6.8) using the dialysis method. The cumulative release of FITC-BSA versus time is plotted in Fig. 7.
 | ||
| Fig. 7 In vitro release of FITC-BSA from nanogels in different buffer solutions (pH 6.8 and pH 7.4) at 37 °C, data represent the average of triplicates. | ||
The release pattern of FITC-BSA loaded nanogels showed an initial burst release for 1 h, and then a continuous and controlled release followed up to 45 h. The initial burst release corresponded to the diffusion of absorbed or loosely encapsulated FITC-BSA in PEG shell of nanogels. During the first 1 h, only 7.2% of FITC-BSA was released at pH 7.4, but 11.8% of FITC-BSA was released at pH 6.8. The cumulative release of FITC-BSA from nanogels at pH 7.4 and pH 6.8 were 24.2% and 40.3%, respectively. The FITC-BSA loaded nanogels showed a more rapid release profile in slightly acidic condition (pH 6.8) in comparison to that in neutral media (pH 7.4). This comparably fast protein release at mildly acidic pH was most probably related to protonation of PLGA block in the core of nanogels which decreased the concentration of negatively charged carboxyl groups. It caused the electrostatic interaction between the patches of positive charge of BSA and PLGA chain was weakened and more incorporated FITC-BSA in the core of nanogels was readily released. Therefore, the core–shell nanogels with acidic accelerated protein release are highly interesting for pH-sensitive protein delivery.
In vitro cytotoxicity assay
The in vitro cytotoxicity of blank nanogels and FITC-BSA loaded nanogels against HeLa cells were determined by the CKK8 assay. Fig. 8 displays the proliferation of HeLa cells after incubation with blank nanogels and FITC-BSA loaded nanogels, respectively. The cell viability of HeLa cells was maintained over 95% with different concentrations of blank nanogels and FITC-BSA loaded nanogels solutions and no toxicity was shown with an increase of the concentration. Therefore, the enzymatically crosslinked nanogels can serve as safe nano-platforms for protein delivery. | ||
| Fig. 8 Viability of HeLa cells after incubation for 24 h with blank nanogels and FITC-BSA loaded nanogels at various specified concentrations. Data presented as average ± stand deviation (n = 3). | ||
Cellular uptake of FITC-BSA-loaded nanogels
The cellular uptake and intracellular protein release behavior of FITC-BSA loaded nanogels was studied using CLSM in HeLa cells. Fig. 9 presents CLSM images of HeLa cells incubated with FITC-BSA loaded nanogels at pH 7.4 or pH 6.8 for 4 h. For comparison, HeLa cells were also incubated with free FITC-BSA for 4 h. HeLa nuclei were stained with Hoechst to provide enhanced contrast and clarity.The results showed that strong FITC fluorescence was observed inside the HeLa cells following 4 h incubation with FITC-BAS loaded nanogels at pH 7.4 and 6.8. It indicated that the FITC-BSA loaded nanogels are taken up by HeLa cells via the endocytic pathway and release the loaded proteins in endosomes. In contrast, little fluorescence was detected in HeLa cells following 4 h treatment with free FITC-BSA due to poor cellular uptake. A relatively high fluorescence signal was observed in Fig. 7c which suggest an enhanced nanogels uptake in HeLa cells incubated at pH 6.8. Compared to nanogels incubated at pH 7.4, the smaller size of nanogels incubated at pH 6.8 results in these nanogels were captured easily by HeLa cells. These results supported that the pH-sensitive PEG-b-P(LGA-g-Tyr) nanogels are able to deliver and release proteins into cancer cells.
Conclusions
In this paper, we developed novel pH-sensitive PEG-b-P(LGA-g-Tyr) nanogels for protein delivery. The core–shell PEG-b-P(LGA-g-Tyr) nanogels were synthesized by a facile enzyme-mediated oxidative coupling reaction in the presence of HRP and H2O2 under physiological condition. The enzymatically crosslinked P(LGA-g-Tyr) core provides excellent stability and pH-responsibility to the nanogels, and the PEG shell improves the biocompatibility and water solubility of nanogels. The enzyme-catalyzed crosslinking technique provides a simple and versatile route for the in situ synthesis of core–shell nanogels with controlled size and morphology. FITC-BSA as a model protein was effectively in situ loaded into the core–shell nanogels with an entrapment efficiency of 69.9%. The release profile of FITC-BSA from PEG-b-P(LGA-g-Tyr) nanogels indicated a small burst release followed by a continued and controlled release. The nanogels showed a distinctly enhanced FITC-BSA release at pH 6.8 compared with that at normal pH 7.4. Cytotoxicity tests with HeLa cells by CCK 8 assay revealed that the PEG-b-P(LGA-g-Tyr) nanogels exhibited good cytocompatibility in vitro. The FITC-BSA loaded nanogels can be effectively taken up by HeLa cells and deliver FITC-BSA to HeLa cells. In conclusion, these new enzymatically crosslinked PEG-b-P(LGA-g-Tyr) nanogels with ideal size and good cytocompatibility offer an interesting potential as a carrier for intracellular protein delivery.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51263017, 51463013 and 51003046).Notes and references
- B. Leader, Q. J. Baca and D. E. Golan, Nat. Rev. Drug Discovery, 2008, 7, 21–39 CrossRef CAS PubMed.
- S. Frokjaer and D. E. Otzen, Nat. Rev. Drug Discovery, 2005, 4, 298–306 CrossRef CAS PubMed.
- T. Vermonden, R. Censi and W. E. Hennink, Chem. Rev., 2012, 112, 2853–2888 CrossRef CAS PubMed.
- Y. Lu, W. Sun and Z. Gu, J. Controlled Release, 2014, 194, 1–19 CrossRef CAS PubMed.
- W. Sun, Y. Lu and Z. Gu, Part. Part. Syst. Charact., 2014, 31, 1204–1222 CrossRef CAS.
- Z. Gu, A. Biswas, M. Zhao and Y. Tang, Chem. Soc. Rev., 2011, 40, 3638–3655 RSC.
- R. T. Chacko, J. Ventura, J. Zhuang and S. Thayumanavan, Adv. Drug Delivery Rev., 2012, 64, 836–851 CrossRef CAS PubMed.
- A. V. Kabanov and S. V. Vinogradov, Angew. Chem., Int. Ed., 2009, 48, 5418–5429 CrossRef CAS PubMed.
- D. M. Eckmann, R. J. Composto, A. Tsourkas and V. R. Muzykantov, J. Mater. Chem. B, 2014, 2, 8085–8097 RSC.
- W. Chen, M. Zheng, F. Meng, R. Cheng, C. Deng, J. Feijen and Z. Zhong, Biomacromolecules, 2013, 14, 1214–1222 CrossRef CAS PubMed.
- D. Lee, K. Choe, Y. Jeong, J. Yoo, S. M. Lee, J. H. Park, P. Kim and Y. C. Kim, RSC Adv., 2015, 5, 14482–14491 RSC.
- L. Milane, S. Ganesh, S. Shah, Z. F. Duan and M. Amiji, J. Controlled Release, 2011, 155, 237–247 CrossRef CAS PubMed.
- L. Tian and Y. H. Bae, Colloids Surf., B, 2012, 99, 116–126 CrossRef CAS PubMed.
- M. Zan, J. Li, S. Luo and Z. Ge, Chem. Commun., 2014, 50, 7824–7827 RSC.
- T. Zhou, C. Xiao, J. Fan, S. Chen, J. Shen, W. Wu and S. Zhou, Acta Biomater., 2013, 9, 4546–4557 CrossRef CAS PubMed.
- N. M. Oh, K. T. Oh, H. J. Baik, B. R. Lee, A. H. Lee, Y. S. Youn and E. S. Lee, Colloids Surf., B, 2010, 78, 120–126 CrossRef CAS PubMed.
- Y. Lee, S. Fukushima, Y. Bae, S. Hiki, T. Ishii and K. Kataoka, J. Am. Chem. Soc., 2007, 129, 5362–5363 CrossRef CAS PubMed.
- U. Hasegawa, S. Sawada, T. Shimizu, T. Kishida, E. Otsuji, O. Mazda and K. Akiyoshi, J. Controlled Release, 2009, 140, 312–317 CrossRef CAS PubMed.
- N. Morimoto, S. Hirano, H. Takahashi, S. Loethen, D. H. Thompson and K. Akiyoshi, Biomacromolecules, 2013, 14, 56–63 CrossRef CAS PubMed.
- A. Shimoda, S. Sawada and K. Akiyoshi, Macromol. Biosci., 2011, 11, 882–888 CrossRef CAS PubMed.
- H. J. Baik, N. M. Oh, Y. T. Oh, N. Y. Yoo, S. Y. Park, K. T. Oh, Y. S. Youn and E. S. Lee, Colloids Surf., B, 2011, 84, 585–590 CrossRef CAS PubMed.
- L. Shi, S. Khondee, T. H. Linz and C. Berkland, Macromolecules, 2008, 41, 6546–6554 CrossRef CAS.
- G. Liu and Z. An, Polym. Chem., 2014, 5, 1559–1565 RSC.
- M. M. Yallapu, M. Jaggi and S. C. Chauhan, Drug Discovery Today, 2011, 16, 457–463 CrossRef CAS PubMed.
- X. Zhang, S. Malhotra, M. Molina and R. Haag, Chem. Soc. Rev., 2015, 44, 1948–1973 RSC.
- L. S. Teixeira, J. Feijen, C. A. van Blitterswijk, P. J. Dijkstra and M. Karperien, Biomaterials, 2012, 33, 1281–1290 CrossRef PubMed.
- R. Jin, C. Hiemstra, Z. Zhong and J. Feijen, Biomaterials, 2007, 28, 2791–2800 CrossRef CAS PubMed.
- M. Kurisawa, J. E. Chung, Y. Y. Yang, S. J. Gao and H. Uyama, Chem. Commun., 2005, 4312–4314 RSC.
- F. Lee, J. E. Chung and M. Kurisawa, Soft Matter, 2008, 4, 880–887 RSC.
- K. Ren, C. He, Y. Cheng, G. Li and X. Chen, Polym. Chem., 2014, 5, 5069–5076 RSC.
- K. M. Park, Y. M. Shin, Y. K. Joung, H. Shin and K. D. Park, Biomacromolecules, 2010, 11, 706–712 CrossRef CAS PubMed.
- M. Kurisawa, F. Lee, L. S. Wang and J. E. Chung, J. Mater. Chem., 2010, 20, 5371–5375 RSC.
- F. Lee, J. E. Chung and M. Kurisawa, J. Controlled Release, 2009, 134, 186–193 CrossRef CAS PubMed.
- S. Sakai and K. Kawakami, J. Biomed. Mater. Res., Part A, 2008, 85, 345–351 CrossRef PubMed.
- K. Xu, F. Lee, S. J. Gao, J. E. Chung, H. Yano and M. Kurisawa, J. Controlled Release, 2013, 166, 203–210 CrossRef CAS PubMed.
- Z. Peng, Y. She and L. Chen, J. Biomater. Sci., Polym. Ed., 2015, 26, 111–127 CrossRef CAS PubMed.
- S. Singh, F. Topuz, K. Hahn, K. Albrecht and J. Groll, Angew. Chem., Int. Ed., 2013, 52, 3000–3003 CrossRef CAS PubMed.
- C. Wu, C. Bottcher and R. Haag, Soft Matter, 2015, 11, 972–980 RSC.
- Y. Wu, Q. Lai, S. Lai, J. Wu, W. Wang and Z. Yuan, Colloids Surf., B, 2014, 118, 298–305 CrossRef CAS PubMed.
- B. Y. Kim, J. W. Bae and K. D. Park, J. Controlled Release, 2013, 172, 535–540 CrossRef CAS PubMed.
- J. Ding, C. Xiao, Y. Li, Y. Cheng, N. Wang, C. He, X. Zhuang, X. Zhu and X. Chen, J. Controlled Release, 2013, 169, 193–203 CrossRef CAS PubMed.
- C. He, C. Zhao, X. Chen, Z. Guo, X. Zhuang and X. Jing, Macromol. Rapid Commun., 2008, 29, 490–497 CrossRef CAS.
- W. Ding, S. Lin, J. Lin and L. Zhang, J. Phys. Chem. B, 2008, 112, 776–783 CrossRef CAS PubMed.
- J. Sun, Y. Huang, Q. Shi, X. Chen and X. Jing, Langmuir, 2009, 25, 13726–13729 CrossRef CAS PubMed.
- Y. Sun, Z. Peng, X. Liu and Z. Tong, Colloid Polym. Sci., 2010, 288, 997–1003 CAS.
- C. Cai, J. Lin, T. Chen, X. S. Wang and S. Lin, Chem. Commun., 2009, 2709–2711 RSC.
- K. Luo, J. Yin, Z. Song, L. Cui, B. Cao and X. Chen, Biomacromolecules, 2008, 9, 2653–2661 CrossRef CAS PubMed.
- S. Sakai and K. Kawakami, Acta Biomater., 2007, 3, 495–501 CrossRef CAS PubMed.
- X. Hu, X. Chen, J. Wei, S. Liu and X. Jing, Macromol. Biosci., 2009, 9, 456–463 CrossRef CAS PubMed.
- H. Wei, C. Cheng, C. Chang, W. Chen, S. Cheng, X. Zhang and R. Zhuo, Langmuir, 2008, 24, 4546–4570 Search PubMed.
- T. F. Yang, C. N. Chen, M. C. Chen, C. H. Lai, H. F. Liang and H. W. Sung, Biomaterials, 2007, 28, 725–734 CrossRef CAS PubMed.
- H. Wei, R. Ravarian, S. Dehn, S. Perrier and F. Dehghani, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 1809–1820 CrossRef CAS.
- Z. Gao and A. Eisenberg, Macromolecules, 1993, 26, 7353–7360 CrossRef CAS.
- H. Maeda, J. Wu, T. Sawa, Y. Matsumura and K. Hori, J. Controlled Release, 2000, 65, 271–284 CrossRef CAS PubMed.
- S. Rosenfeldt, A. Wittemann, M. Ballauff, E. Breininger, J. Bolze and N. Dingenouts, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2004, 70, 061403 CrossRef CAS PubMed.
- S. Salmaso and P. Caliceti, Int. J. Pharm., 2013, 440, 111–123 CrossRef CAS PubMed.
- A. Wittemann, B. Haupt and M. Ballauff, Phys. Chem. Chem. Phys., 2003, 5, 1671–1677 RSC.
- A. Wittemann and M. Ballauff, Phys. Chem. Chem. Phys., 2006, 8, 5269–5275 RSC.
- K. Henzler, B. Haupt, S. Rosenfeldt, L. Harnau, T. Narayanan and M. Ballauff, Phys. Chem. Chem. Phys., 2011, 13, 17599–17605 RSC.
- J. M. Park, B. B. Muhoberac, P. L. Dubin and J. Xia, Macromolecules, 1992, 25, 290–295 CrossRef CAS.
- T. Matsudo, K. Ogawa and E. Kokufuta, Biomacromolecules, 2003, 4, 1794–1799 CrossRef CAS PubMed.
- R. Sjöback, J. Nygren and M. Kubista, Spectrochim. Acta, Part A, 1995, 51, L7–L21 CrossRef.
- M. Hartlieb, D. Pretzel, M. Wagner, S. Hoeppener, P. Bellstedt, M. Görlach, C. Englert, K. Kempe and U. S. Schubert, J. Mater. Chem. B, 2015, 3, 1748–1759 RSC.
- Y. Zhang, K. Han, D. Lu and Z. Liu, Soft Matter, 2013, 9, 8723–8729 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra25133h |
| This journal is © The Royal Society of Chemistry 2016 |