A new process for enhancing conversion of methyl vinyl ether to propylene yields with Al2O3 hollow spheres
Yang Wanga,
Wenyu Zhanga,
Daoan Zhab,
Jiaji Hua,
Wei Lic,
Wubiao Duana and
Bo Liu*a
aDepartment of Chemistry, School of Science, Beijing Jiaotong University, Beijing 100044, China. E-mail: boliu@bjtu.edu.cn
bState Key Laboratory of Solid Waste Reuse for Building Materials, Beijing Building Materials Academy of Science Research, Beijing, 100041, China
cPetrochina Kunlun Gas Co., Ltd Jilin Branch, Jilin, 132000, China
First published on 6th January 2016
Abstract
Al2O3 hollow spheres were successfully synthesized via a simple template method and used to catalyze a new process from methyl vinyl ether to propylene. In this study, it not only indicated that a two-step integration process from acetylene and methanol to propylene is feasible but the prepared catalyst could also adjust the distribution of the products. First, colloidal carbon spheres were formed by the hydrothermal synthesis method, and then the template was used to prepare hollow spheres of Al2O3. EDS, XRD, SEM, and TEM all showed that the catalyst was composed of only O and Al and the structures were hollow spheres. In the new process tests, the low carbon olefins are detected as the main products, alkanes are nearly not observed. The participation of Al2O3 hollow spheres could significantly enhance the selectivity of olefins especially for propylene compared to the results without catalysts. Furthermore, the effects of temperature on the products were also investigated, and a possible radical-involved reaction mechanism is discussed in detail at the end.
Introduction
Light olefins such as ethylene, propylene and butylene are valuable feedstocks for the petrochemical industry,1,2 especially propylene, which can be used in the manufacture of polypropylene, acrylonitrile, propylene oxide, cumene and acrylic acid, etc. The growth rate of propylene demand is estimated at 4–5% per year.3,4 Currently, about 70% of worldwide propylene production comes from steam crackers and 28% from refinery fluid catalytic cracking (FCC) units.5 However, the petroleum route is unable to meet the increasing demand of propylene; therefore, various types of new processes for preparing propylene from cost-effective resources have been developed as alternatives to traditional petroleum decomposition. Producing propylene through simple raw materials has gradually become a hot topic, for example, the conversion of methanol to propylene,6,7 and dehydrogenation of propane.8–11 Taoufik et al. reported a new and efficient catalytic reaction that can directly develop ethylene into propylene with selectivity higher than 95%.12 He et al. used CH4 as the starting material and disclosed a two-step reaction route catalyzed by modified CeO2 nanocrystals, and achieved the conversion of CH4 to propylene via monohalogenomethanes.13Acetylene, as one of the most common chemical raw materials, receives continuous attention as well. Many researchers have been trying to integrate acetylene and other small molecular compounds to produce propylene. In early years, Belavin et al. investigated the feasibility of the reaction of acetylene and methane to form propylene in one step. However, they failed to realize this using Fe, Co, Ni and Cr2O3 as the catalyst and concluded that the one step reaction is not feasible.14 Afterwards, Yang et al. disclosed an approach to produce propylene using acetylene and carbon dioxide as the materials, a re-modeled nitrogenase was used to catalyze the reaction. Findings suggested that when CO2 was used as the substrate, propylene was detected as the major product after adding the appropriate amount of acetylene.15 The integration of acetylene and carbon monoxide was also studied. Zhang et al. used the Fischer–Tropsch synthesis and they illustrated that with the participation of carbon monoxide, acetylene can form ethylene, propylene and butylene.16 In our study, the new integrated reaction of acetylene and methanol to form propylene was proposed.
As reported,17–19 acetylene reacts with methanol under alkaline conditions at below 300 °C and could produce methyl vinyl ether (MVE), which is a kind of widely used industrial material. The researchers first found that MVE monomer is easy to polymerize at low temperatures and the polymers have good physicochemical properties that could be applied in the fields of adhesives,20,21 plasticizers,22 pesticides,23 biomedical materials,24 dielectric materials25 and UV curing.26 Due to the characteristic of easy polymerization at low temperatures, the subjects almost always focused on MVE as a precursor for polymer preparation. However, the catalytic properties of MVE at high temperatures were greatly ignored. As far as we know, no research group has reported the products of MVE via a catalytic process at high temperatures. As an intermediate from acetylene and methanol, it is reasonable to think it is feasible to generate propylene through MVE. In our previous study, the joint reaction of methanol and MVE on Ba/ZSM-5 has already been investigated, and we have found the potential of MVE for production of olefins.27
In this study, we propose a feasible process with acetylene and methanol as the starting materials to produce propylene in two steps. The intermediate MVE is generated in the first step, and is then further converted to propylene. Herein, the second-step conversion from MVE to propylene is our major concern. At first, we show that propylene can be produced by thermal cracking of MVE directly without using any catalysts. This process is greatly simplified; the products in the pyrolysis reaction are alkenes and aldehydes that are easily separated when compared to the MTP method. Moreover, Al2O3 hollow spheres were used in order to enhance the selectivity of propylene. These results showed that the yield of propylene could increase nearly three times. The mechanism is also proposed to explain the data we got in the end.
Experimental
Preparation of colloidal carbon spheres
In the synthesis of colloidal carbon spheres,28 60 g of glucose (C6H12O6·H2O) was dissolved in 300 mL of deionized water. The resultant solution was transferred to a 500 mL Teflon-sealed autoclave and maintained at 180 °C for 12 h. Subsequently, the dark brown liquid products were washed with ethanol and deionized water several times using centrifugal treatment until the filtrate become colorless. Finally, the solid products were dried at 80 °C for 12 h and grounded to a powder for use.Preparation of Al2O3 hollow spheres
45 g of Al(NO3)3·9H2O was dissolved in 300 mL of anhydrous ethanol, then 1 g of the prepared carbon spheres were added into the solution followed by ultrasonic dispersion for 2 h to become a brown mixture. Then, the mixture was placed at room temperature for 24 h. The obtained product was washed with deionized water repeatedly and dried at 80 °C for 6 h. The final dried powder product was calcined at a ramp heating rate of 3 °C min−1 in static air from room temperature to 500 °C and maintained at 500 °C for 4 h.Catalyst characterization
Powder X-ray diffraction (Rigaku Ultima IV) was used to characterize the crystallinity and chemical composition of the materials studied. The intensity data was collected over a 2θ range of 10–80°. The step size was 0.025° with a time duration per step of 0.8 s. The surface structure and EDS data of the Al-based materials were characterized using SEM (HITACHI SU8010). The samples were placed on a double-sided carbon tape mounted on a sample holder, subsequently coated for 30 s using a gold semi-high resolution coater. Transmission electron microscopy (TEM) was employed to gain insights into the detailed structure of the sorbent powders. The BET analysis of the sample was obtained using Micromeritics Instrument Corporation Tristar II 3020 at 77 K. The pore diameters were determined by means of the BJH method.Pyrolysis experiments
Methyl vinyl ether is employed as the only starting material. Experiments were performed in a clean and dry quartz tube furnace, the volume of the tube was 1 L. Before the experiments, the reactor was purged with nitrogen to exhaust air for 30 min, then the furnace temperature was pre-set at certain values (200 °C, 300 °C, 350 °C, 400 °C, 500 °C, 600 °C and 700 °C). The weight hourly space velocity (WHSV) was controlled at 1.2 L h−1 under atmospheric pressure, and the gaseous reactant methyl vinyl ether was added to the reactor when the temperature was stable. When the reaction was stable, the gaseous product was collected with a gas collection pocket of aluminum foil. After sealing, the samples were examined by GC-MS detection (see Fig. 1). | ||
| Fig. 1 Apparatus for catalytic reactions. (1) High-purity nitrogen (2) methyl vinyl ether (3) tubular furnace (4) fixed bed reactor (5) gas–liquid separator (6) GC-MS (7) shut-off valve. | ||
Catalytic experiments
The prepared Al2O3 hollow spheres were mixed with an appropriate amount of quartz sand fill in the middle of the reactor. Other operating conditions are identical to the pyrolysis experiments. The temperatures were set at 250 °C, 350 °C, 400 °C, 500 °C, 600 °C and 700 °C, respectively.Results and discussion
Characteristics of catalysts
EDS analysis (Fig. 2(a)) showed that the prepared sample has two peaks which represent O and Al, respectively. The C element was not detected, which means the template was removed completely after calcination. As shown in Fig. 2(b), no strong diffraction peaks appear, indicating that the catalysts are in a form of agraphitic Al2O3. | ||
| Fig. 2 Structure features of alumina hollow spheres (a) EDS spectrum of alumina hollow spheres (b) XRD pattern of alumina hollow spheres. | ||
The morphology of colloidal carbon spheres and Al2O3 hollow spheres were shown as Fig. 3 and 4. Due to the abundant –OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O on the colloidal carbon spheres external surface, Al3+ would be easy to adsorb on the surface of the template until the thickness of the adsorbed layer reaches a certain value and the balance was done. For the template removal process, carbon will transform to CO2 in the air atmosphere leaving a hollow structure. Obviously, most Al2O3 hollow spheres keep their shape intact, the hollow structure could be seen from TEM and by the presence of ruptured hollow spheres. Due to the process of removing the template, the volume of the spheres shrunk with the release of CO2, compared to colloidal carbon, the surface of the catalysts was not smooth and all of them kept a folded surface. The BET surface area and pore size distribution of the two samples are presented in Fig. 5(a)–(d). The BET surface areas of the Al2O3 hollow spheres was up to 194 m2 g−1, which was larger than the commercial γ-Al2O3 (about 120 m2 g−1). Moreover pore size distribution (PSD) analysis indicates that these spheres have average pore diameters of 12.3 nm, which was conducive to absorbing reactants into the hollow structure compare to 6.1 nm for γ-Al2O3.
O on the colloidal carbon spheres external surface, Al3+ would be easy to adsorb on the surface of the template until the thickness of the adsorbed layer reaches a certain value and the balance was done. For the template removal process, carbon will transform to CO2 in the air atmosphere leaving a hollow structure. Obviously, most Al2O3 hollow spheres keep their shape intact, the hollow structure could be seen from TEM and by the presence of ruptured hollow spheres. Due to the process of removing the template, the volume of the spheres shrunk with the release of CO2, compared to colloidal carbon, the surface of the catalysts was not smooth and all of them kept a folded surface. The BET surface area and pore size distribution of the two samples are presented in Fig. 5(a)–(d). The BET surface areas of the Al2O3 hollow spheres was up to 194 m2 g−1, which was larger than the commercial γ-Al2O3 (about 120 m2 g−1). Moreover pore size distribution (PSD) analysis indicates that these spheres have average pore diameters of 12.3 nm, which was conducive to absorbing reactants into the hollow structure compare to 6.1 nm for γ-Al2O3.
 | ||
| Fig. 3 SEM images of the prepared catalysts (a) and (b) colloidal carbon spheres (c) and (d) (e) hollow spheres Al2O3. | ||
The effects of temperature in pyrolysis experiments
First, the decomposition and the selectivity rate of propylene by thermal cracking MVE were studied in different temperatures. The pyrolysis products include ethylene, propylene, isobutylene, acetaldehyde and propanal. Table 1 shows the content of each component in the mixture. It can be found that the selectivity of propylene and the conversion rate of MVE were low, but still show very gradual increments as the temperature rose until 400 °C. When the temperature stayed higher than 400 °C, both the selectivity and the conversion rate are improved dramatically. The maximum selectivity of propylene is around 13.8% at 600 °C (see Fig. 6).| CO [%] | C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) a [%] a [%] |
C3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) b [%] b [%] |
C4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) c [%] c [%] |
CH3CHO [%] | CH3CH2CHO [%] | MVEd [%] | |
|---|---|---|---|---|---|---|---|
a C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) represents ethylene.b C3 represents ethylene.b C3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) represents propylene.c C4 represents propylene.c C4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) represents isobutene.d MVE represents methyl vinyl ether. represents isobutene.d MVE represents methyl vinyl ether. |
|||||||
| 200 °C | 1.7 | — | — | — | — | — | 68.5 |
| 300 °C | 2.3 | — | — | — | — | — | 64.7 |
| 350 °C | 8.2 | — | 0.06 | 0.01 | 0.08 | 0.01 | 61.3 |
| 400 °C | 10.8 | — | 0.4 | 0.03 | 0.14 | 0.02 | 58.5 |
| 500 °C | 17.0 | 13.0 | 13.0 | 3.5 | 2.8 | 1.4 | 46.1 |
| 600 °C | 39.7 | 14.4 | 13.8 | 2.7 | 1.5 | 0.3 | — |
| 700 °C | 43.1 | 27.3 | 12.0 | 0.2 | 0.3 | — | — |
The effects of Al2O3 hollow spheres as catalysts
Al2O3 hollow spheres have not only high specific surface areas but could keep stable at higher temperatures and was regarded as good catalysts according to the proposed mechanism below. We found the catalyst's participation can significantly adjust the distribution of the products. Based on Al2O3 hollow spheres, the pyrolysis at different temperatures (250 °C, 350 °C, 400 °C, 500 °C, 600 °C and 700 °C) was conducted. As a contrast, commercial Al2O3 with lower a BET surface (120 m2 g−1) was also tested (presented in Fig. 8). Table 2 showed the operating conditions and the main products. The comparison on the selectivity of ethylene and propylene for the two tests is shown as Fig. 7(a), the catalyst obviously enhanced the olefin yield even at lower temperatures. However, the conversion of MVE is the same up to 99% in the higher temperatures that are shown in Fig. 7(b).| Temperature (°C) | Pressure (MPa) | WHSVa (L h−1) | Selectivity of ethylene [%] | Selectivity of propylene [%] | Conversion of MVE [%] |
|---|---|---|---|---|---|
| a Weight hourly space velocity. | |||||
| 250 °C | 0.3 | 1.2 | — | 0.4 | 41.0 |
| 350 °C | 0.3 | 1.2 | 15.4 | 17.2 | 98.8 |
| 400 °C | 0.3 | 1.2 | 15.2 | 26.5 | >99.0 |
| 500 °C | 0.3 | 1.2 | 13.1 | 29.8 | >99.9 |
| 600 °C | 0.3 | 1.2 | 23.5 | 40.0 | >99.9 |
| 700 °C | 0.3 | 1.2 | 17.8 | 2.0 | >99.9 |
Fig. 7(c) and (d) illustrated the differences of C3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) /C2
/C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) and C3
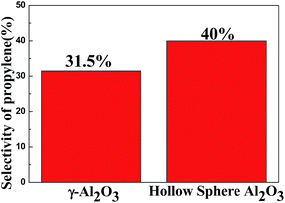
and C3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) yield between catalytic and pyrolysis tests. In the pyrolysis experiments, the highest yield of propylene is 13.8% at 600 °C and was 31.5% when catalysed with commercial Al2O3 (Fig. 8). However, it could reach 40% at 600 °C with Al2O3 hollow spheres, triple the yield in addition to the enhanced selectivity of ethylene. Moreover, C3
yield between catalytic and pyrolysis tests. In the pyrolysis experiments, the highest yield of propylene is 13.8% at 600 °C and was 31.5% when catalysed with commercial Al2O3 (Fig. 8). However, it could reach 40% at 600 °C with Al2O3 hollow spheres, triple the yield in addition to the enhanced selectivity of ethylene. Moreover, C3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) /C2
/C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) in the catalytic test is higher than the pyrolysis test, indicating the selectivity of propylene increased.
in the catalytic test is higher than the pyrolysis test, indicating the selectivity of propylene increased.
The proposed mechanism
Blades et al. had investigated the thermal decomposition of vinyl ethyl ether into ethylene and acetaldehyde; however, they failed to establish a mechanism for the reaction.29 Lauer et al. found the thermal decomposition of phenyl vinyl ether at 260–300 °C produces phenol, acetaldehyde diphenylacetal and an unknown substance of high molecular weight.30 Murad reported the photolysis of ethyl vinyl ether and suggested that some radical combination reactions are responsible for the formation of the products.31 Herein, the mechanism of MVE catalyzed by Al2O3 hollow spheres is proposed in Fig. 9.Actually, the catalysis process is regarded to be accomplished in two steps. In the first step, it is believed the pyrolysis on Al2O3 is the main process and the active intermediate is playing a vital role in the propylene and other by-products formation processes. By analyzing the product components at different temperatures, the formation of the active intermediates is proposed. In the second step, the thermal cracking mechanism of MVE is suggested as below in eqn (1).
 | (1) |
In this step, there is a competition between propylene formation and ethylene formation. Propylene only comes from the intermediate CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH upon combination with CH3 free radicals. Generally, there are three ways of generating ethylene. The first way results from the reaction between CH2
CH upon combination with CH3 free radicals. Generally, there are three ways of generating ethylene. The first way results from the reaction between CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH and H. The second is the dehydration of dimethyl ether. The last way is the cracking of butylenes. See eqn (2).
CH and H. The second is the dehydration of dimethyl ether. The last way is the cracking of butylenes. See eqn (2).
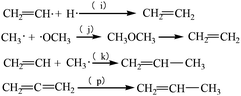 | (2) |
Competition also exists between the formation reactions of acetaldehyde and propanal. Actually, the formation of acetaldehyde and propanal is due to the reactions of CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O with H and CH3 respectively. The reaction mechanisms are shown in eqn (3).
O with H and CH3 respectively. The reaction mechanisms are shown in eqn (3).
 | (3) |
Formation of the two isomers of butylene is more complicated and very different from the above. We suggest that the participation of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 plays an important role. CH2
CH2 plays an important role. CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 undergoes cycloaddition with methylene carbene. Then, the product will continue to react and generate two kinds of butene via a ring-opening addition reaction due to the massive amount of H in the mixture. See eqn (4).
CH2 undergoes cycloaddition with methylene carbene. Then, the product will continue to react and generate two kinds of butene via a ring-opening addition reaction due to the massive amount of H in the mixture. See eqn (4).
 | (4) |
In order to confirm the mechanism, a series of experiments were done. According to the mechanism, temperature was the key factor that could significantly affect the distribution of the products. For example, when the temperature was low, there must be some intermediates that couldn't be observed at higher temperatures due to their instability. Therefore, the mechanism could be confirmed by correctly predicting the products under various temperatures. Therefore, the trace products were exhaustively investigated from 200 °C to 700 °C. As expected, the products did include acetylene, dimethyl ether, methanol and water etc. that were shown in Table 3. The reasons are discussed in detail below.
| Entry | CH3OCH3 [%] | C–C [%] | H2O [%] | CH3OH [%] | C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C [%] C–C [%] |
C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C [%] C [%] |
|---|---|---|---|---|---|---|
| 200 °C | 0.1 | — | 0.1 | 0.1 | — | 26.3 |
| 300 °C | 0.1 | — | 0.04 | 0.1 | — | 28.3 |
| 350 °C | 0.5 | — | 0.04 | 0.1 | — | 30.7 |
| 400 °C | 0.3 | — | 0.1 | 0.1 | — | 27.4 |
| 500 °C | 0.2 | — | — | — | — | — |
| 600 °C | — | 8.3 | — | — | — | — |
| 700 °C | — | 11.8 | — | — | 3.0 | — |
When the temperature was below 400 °C, the predicted products of dimethyl ether and acetylene did exist, especially the amount of acetylene was very large, which was highly in accordance with the theory. Due to the changes in active intermediates generated in the pyrolysis of MVE with temperature, the MVE decomposition rate is low as the temperature didn't come to the pyrolysis point, and at this condition, process (a) dominates with small amounts of CH3 radicals being generated. The main product in this process is OCH3. Therefore, it is very difficult to form propylene (below 1%, as shown in Table 1). Another important evidence was the significant reduction of acetylene when the temperature was above 400 °C, which could be exactly predicted by process (c). The generated hydrogen took part in the formation of olefins that lead to the dramatic increase in olefin yields as shown in Table 1. In this condition, process (d) and (e) occur dominantly while process (b) is greatly suppressed, which results in the significant reduction of acetylene.
However, the routes to generate propylene were limited as described in eqn (2). Considering the competition between ethylene and propylene, as a consequence, the key to enhance the selectivity was to make process (d) dominant. In other words, make MVE one-step dehydration seem imperative. Therefore, Al2O3 hollow spheres with large BET surfaces were chosen. During the reaction, MVE will absorb on Al2O3. Due to the reaction between solid and liquid, the contact between the reactants was very imperative. The hollow structures with pores formed by the release of CO2 (Fig. 3(d) and 4) were more likely to capture the reactant molecules and cause the time of the reactants on the catalyst to be longer compared to the irregularly shaped morphology of commercial Al2O3. Therefore, the reaction would be more efficient. Moreover, Al2O3 combined with H2O was considered as an exothermic reaction, which made the reaction tend to be stronger. As predicted, the yield of propylene increased significantly (Table 2). It is because Al2O3 hollow spheres made the dehydration of MVE to form CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH
CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 become more desirably. Simultaneously, the sharp drop of acetylene can lead to an increase of H, which is great for producing propylene.
CH2 become more desirably. Simultaneously, the sharp drop of acetylene can lead to an increase of H, which is great for producing propylene.
Conclusions
We have shown that a catalyst-free approach to produce propylene from MVE is achievable. The two-step process, from acetylene and methanol to MVE followed by conversion to propylene, is feasible. It was found that the products of the pyrolysis process are closely connected with temperatures; the selectivity of propylene is up to 13.8% when the temperature reaches 600 °C. The pyrolysis mechanism is also proposed and involves radical reactions. Furthermore, we have found that the distribution of the products can be significantly altered by using some dehydrating agents, especially for enhancing the yield of propylene. Using Al2O3 hollow spheres as the dehydrating agent, the selectivity of propylene is enhanced by 40%. Compared to the traditional way to produce propylene, the products in our process are much easier to separate and purify, which will significantly reduce operational costs. The exact mechanism of the catalytic reactions and the effect of other catalysts are still under investigation in our lab.References
- A. S. Al-Dughaither and H. I. Lasa, Fuel, 2014, 138, 52–64 CrossRef CAS.
- D. L. Sun, Y. Yamada and S. Sato, Appl. Catal., B, 2015, 174, 13–20 CrossRef.
- M. A. B. Siddiqui, A. M. Aitani, M. R. Saeed, N. Al-Yassir and S. Al-Khattaf, Fuel, 2011, 90, 459–466 CrossRef CAS.
- S. A. Al-Ghamdi and H. I. Lasa, Fuel, 2014, 128, 120–140 CrossRef CAS.
- K. Hao, B. J. Shen, Y. D. Wang and J. Ren, J. Ind. Eng. Chem., 2012, 18, 1736–1740 CrossRef CAS.
- J. F. Haw, W. Song, D. M. Marcus and J. B. Nicholas, Acc. Chem. Res., 2003, 36, 317–326 CrossRef CAS PubMed.
- M. Stöcker, Microporous Mesoporous Mater., 1999, 29, 3–48 CrossRef.
- M. Razavian and S. Fatemi, Microporous Mesoporous Mater., 2015, 201, 176–189 CrossRef CAS.
- F. Jiang, L. Zeng, S. R. Li, G. Liu, S. P. Wang and J. L. Gong, ACS Catal., 2015, 5, 438–447 CrossRef CAS.
- P. Andy and M. E. Davis, Ind. Eng. Chem. Res., 2004, 43, 2922–2928 CrossRef CAS.
- D. Akporiaye, S. F. Jensen, U. Olsbye, F. Rohr, E. Rytter, M. Rønnekleiv and A. I. Spjelkavik, Ind. Eng. Chem. Res., 2001, 40, 4741–4748 CrossRef CAS.
- M. Taoufik, E. L. Roux, J. T. Cazat and J. M. Basset, Angew. Chem., Int. Ed., 2007, 46, 7202–7205 CrossRef CAS PubMed.
- J. L. He, T. Xu, Z. H. Wang, Q. H. Zhang, W. P. Deng and Y. Wang, Angew. Chem., Int. Ed., 2012, 51, 2438–2442 CrossRef CAS PubMed.
- B. V. Belavin, Neftekhimiya, 1989, 6, 746–750 Search PubMed.
- Z. Y. Yang, V. R. Moure, D. R. Dean and L. C. Seefeldt, Proc. Natl. Acad. Sci. U. S. A., 2012, 48, 19644–19648 CrossRef PubMed.
- R. L. Zhang, X. Hao, Y. Yang and Y. W. Li, Catal. Commun., 2011, 12, 1146–1148 CrossRef CAS.
- J. C. Ghosh, S. K. Bhattacharyya and D. K. R. Chandhufi, Petroleum, 1982, 19, 358 Search PubMed.
- S. Dirk, M. Holger and R. Thomas, J. Organomet. Chem., 1991, 414, C45 CrossRef.
- A. Kyoji and H. Hideki, Japan Patent 4095040, 1990.
- S. Y. Moon and C. M. Chung, J. Macromol. Sci., Part A: Pure Appl. Chem., 2001, 38, 243–252 CrossRef.
- S. A. Madbouly and T. Ougizawa, J. Macromol. Sci., Part B: Phys., 2004, 43, 471–487 CrossRef.
- T. Namikoshi, T. Hashimoto and M. Urushisaki, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 4389–4393 CrossRef CAS.
- E. R. Kenawy and M. A. Sakran, J. Appl. Polym. Sci., 2001, 80, 415–421 CrossRef CAS.
- V. K. Sachan, A. Devi, R. S. Katiyar, R. K. Nagarale and P. K. Bhattacharya, Eur. Polym. J., 2014, 56, 45–58 CrossRef CAS.
- K. Su, D. R. Bujalski, K. Eguchi, G. V. Gordon, S. L. Hu and D. L. Ou, J. Mater. Chem., 2005, 15, 4115–4124 RSC.
- G. K. Noren, ACS Symp. Ser., 1997, 673, 121–132 CrossRef CAS.
- Y. Wang, G. X. Jian, Z. H. Peng, J. J. Hu, X. Wang, W. B. Duan and B. Liu, Catal. Commun., 2015, 66, 34–37 CrossRef CAS.
- M. B. Zheng, J. M. Cao, X. Chang, J. Wang, J. S. Liu and X. J. Ma, Mater. Lett., 2006, 60, 2991–2993 CrossRef CAS.
- A. T. Blades and G. W. Murphy, J. Am. Chem. Soc., 1952, 74, 1039–1041 CrossRef CAS.
- W. M. Lauer and M. A. Spielman, J. Am. Chem. Soc., 1933, 55, 1572–1574 CrossRef CAS.
- E. Murad, J. Am. Chem. Soc., 1961, 83, 1327–1330 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |