Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Low temperature sintering process of copper fine particles under nitrogen gas flow with Cu2+-alkanolamine metallacycle compounds for electrically conductive layer formation†
Tetsu Yonezawa*,
Hiroki Tsukamoto,
Yingqiong Yong,
Mai Thanh Nguyen and
Masaki Matsubara‡
Division of Materials Science and Engineering, Faculty of Engineering, Hokkaido University, Kita 13 Nishi 8, Kita-ku, Sapporo, Hokkaido 060-8628, Japan. E-mail: tetsu@eng.hokudai.ac.jp
First published on 18th January 2016
Abstract
A novel low cost sintering process of copper fine particles to a copper conductive layer was demonstrated at as low as 100 °C without reductive gas flow. Sintering of a mixture of copper particles and copper-based metal–organic-decomposition (MOD) ink gave a copper film with high packing density and low resistivity (9 × 10−6 Ω m). This novel process may open a new strategy in the field of printed electronics.
Introduction
The unique properties and applications of metal nanoparticles and fine particles have driven the development of recent materials.1–5 Many efforts have been made to develop low-temperature sintering processes for printed electronics, especially for organic devices as well as printing on conventional polymer films. Printable processes using nanomaterials are expected to open a door for novel manufacturing processes which dramatically decreases the energy consumption compared to conventional processes. Metallic silver inks and films with high stability and a high conductivity are one of the most representative materials of the printed electronics technology. However, silver ion migration is an old problem which still needs to be solved. Metallic copper nanoparticles and fine particles are considered as a preferable candidate according to their low cost, high conductivity, and lower electromigration.5–11 However, their easy oxidation has prevented their practical use, especially for the low temperature sintering process.In order to overcome the problems of copper, Yabuki12 and our group13–16 have recently studied low temperature sintering processes using a two-step strategy, i.e. oxidative annealing and reductive annealing. This two-step sintering process gave a very low sintering temperature to submicron copper fine particles. At present, the sintering temperature is still a little high (150 °C,15 200 °C16,17 or more than 200 °C13,14,18). We used a proton-initiating decomposable polymer as the stabilizing reagent for copper fine particles and they were sintered at 150 °C.19 In order to apply copper inks or pastes to organic electronic devices, very low sintering temperatures are demanded. For example, laser sintering is one possibility for low temperature sintering.20,21
Recently, metal–organic-decomposition (MOD) ink has attracted considerable attention for low temperature sintering. The MOD ink of copper consists of copper salts, ligands, and some additives; this ink will decompose to form a copper film after sintering.22–26 Kim et al.26 demonstrated that the ultimate electrical resistivity of a film depends on the copper concentration in a copper(II) formate (CuF)–hexylamine complex which controls the porosity and impurity content of the film. The lowest electrical resistivity, obtained from the ink (mole ratio of Cu to hexylamine was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6) annealed at 200 °C followed by formic acid reduction at 250 °C, was 5.2 × 10−8 Ω m. Yabuki and coworkers used complexes of copper formate and various amines, and studied the relationship between the size of nanoparticles and the types of amines as well as the length of the alkyl chain. The lowest resistivity they could achieve was 5 × 10−8 Ω m at 140 °C.25 Shin et al. reported a self-reducible and alcohol-soluble copper-based MOD ink. They used CuF as the precursor, 2-amino-2-methyl-1-propanol as the ligand, octylamine as the co-complexing agent, and hexanoic acid as the sintering helper to obtain a resistivity of 2.3 × 10−7 Ω m after sintering at 200 °C under N2.22 Farraj et al. investigated the mechanism of the decomposition process of the complex between copper formate and 2-amino-2-methyl-1-propanol. The lowest resistivity after sintering under N2 was 1.1 × 10−7 Ω m at 190 °C.23 Choi et al. elucidated the nucleation and growth behavior of copper nanoparticles which determined microstructure of film during sintering. Using copper(II) formate as the copper source and hexylamine as the ligand, they achieved the lowest resistivity of the copper film (4.1 × 10−7 Ω m) at 200 °C under a formic acid atmosphere.24
2.6) annealed at 200 °C followed by formic acid reduction at 250 °C, was 5.2 × 10−8 Ω m. Yabuki and coworkers used complexes of copper formate and various amines, and studied the relationship between the size of nanoparticles and the types of amines as well as the length of the alkyl chain. The lowest resistivity they could achieve was 5 × 10−8 Ω m at 140 °C.25 Shin et al. reported a self-reducible and alcohol-soluble copper-based MOD ink. They used CuF as the precursor, 2-amino-2-methyl-1-propanol as the ligand, octylamine as the co-complexing agent, and hexanoic acid as the sintering helper to obtain a resistivity of 2.3 × 10−7 Ω m after sintering at 200 °C under N2.22 Farraj et al. investigated the mechanism of the decomposition process of the complex between copper formate and 2-amino-2-methyl-1-propanol. The lowest resistivity after sintering under N2 was 1.1 × 10−7 Ω m at 190 °C.23 Choi et al. elucidated the nucleation and growth behavior of copper nanoparticles which determined microstructure of film during sintering. Using copper(II) formate as the copper source and hexylamine as the ligand, they achieved the lowest resistivity of the copper film (4.1 × 10−7 Ω m) at 200 °C under a formic acid atmosphere.24
In the above reports, the sintering temperature (130–250 °C) to achieve a low resistivity in the range of 10−6 to 10−8 Ω m is still high from a cost perspective.
In this study, we would like to propose a novel method to obtain a high conductivity copper film at low sintering temperature based on the addition of copper particles to a complex of copper(II) formate and 1-amino-2-propanol (isopropanolamine) to form the copper ink. We expect a higher packing density of the copper film after decomposition of copper complex followed by the co-sintering of the added copper and generated copper particles, leading to a higher conductivity at a lower sintering temperature. Our results demonstrated that without using any organic additives and helpers as well as reductive gas during sintering, we successfully obtained copper films with resistivity in the order of magnitude of 10−6 Ω m at 100 °C. To the best of our knowledge, this is the first time that ink is prepared by mixing copper particles with copper-based MOD ink and that such low resistivity is obtained at 100 °C by using a copper-based MOD ink.
Experimental
Materials
Copper(II) formate tetrahydrate ((HCOO)2Cu·4H2O, CuF) (Wako, Japan) was used as copper source. Alkanolamines including 1-amino-2-propanol (isopropanolamine, IPA, Junsei, Japan), 2-amino-1-butanol (2AB, TCI), and 2-diethylaminoethanol (DEAE, Junsei) were used as the ligands to form the complexes with CuF. Water was purified using an Organo/ELGA Purelab system (>0.18 MΩ m). Copper fine particles (Dowa, Japan) with median diameters of 0.8 μm (determined by SEM, Fig. S1†) were used as received.Preparation of copper(II) formate–alkanolamine (CuF–alkanolamine) inks
We have selected the aforementioned alkanolamines as ligands because stable, 5-membered metallacycles can be formed between the alkanolamines and copper. 10 mmol of CuF (2.26 g) was added to 10 mmol of the alkanolamines (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; mol CuF
1; mol CuF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol alkanolamine), IPA (0.75 g), 2AB (0.89 g), and DEAE (1.17 g). These alkanolamines are bidentate. The chemical structures of the obtained CuF–alkanolamine complexes are displayed in Fig. 1. Then, the mixture was stirred to obtain homogeneous liquids. After CuF completely dissolved in the alkanolamines, the copper complexes were used as the MOD inks to prepare the copper films.
mol alkanolamine), IPA (0.75 g), 2AB (0.89 g), and DEAE (1.17 g). These alkanolamines are bidentate. The chemical structures of the obtained CuF–alkanolamine complexes are displayed in Fig. 1. Then, the mixture was stirred to obtain homogeneous liquids. After CuF completely dissolved in the alkanolamines, the copper complexes were used as the MOD inks to prepare the copper films.
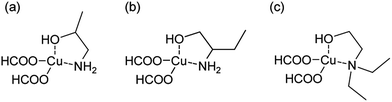 | ||
| Fig. 1 Chemical structures of copper–alkanolamine complexes studied here. (a) CuF–IPA, (b) CuF–2AB, and (c) CuF–DEAE. | ||
Preparation of copper(II) formate–IPA–copper particle (CuF–IPA–Cu) inks
CuF–IPA complexes were prepared using CuF (10 mmol, 2.26 g) and IPA (10 mmol, 0.75 g). Copper particles with median diameters of 0.8 μm (Fig. S1†) were added into the as-prepared Cu–IPA complexes using a molar ratio of 1 to 6 (CuF to copper particle), followed by mixing using a conditioning mixer less than 30 min to form the CuF–IPA–Cu inks. The total concentration of Cu in the ink was approximately 65 wt%.Preparation of copper films
The as-prepared CuF–alkanolamine inks and CuF–IPA–Cu inks were deposited on alumina substrates using a doctor blade (used thicknesses of the printed areas were 100 μm and 40 μm, respectively) and sintered in a tube furnace at 100 °C under N2 gas at a flow rate of 1 dm3 min−1. 99.99% N2 gas from a cylinder was used for sintering the CuF–alkanolamine inks, and 99.9% N2 gas generated using a nitrogen supplier (model 05, System Instruments, Japan) was used for sintering the CuF–IPA–Cu ink. The obtained copper layers were characterized and used for further measurements.Characterizations and measurements
Thermogravimetric-differential thermal analysis (TGA-DTA) measurements were performed using a Shimadzu DTG-60H. X-ray diffraction (XRD) patterns were obtained using a Rigaku Miniflex-II diffractometer. Scanning electron microscopy (SEM) images of the copper particles and copper films were collected using a JEOL JSM-6701F field-emission type SEM. The resistivity of copper films was measured by a four-point probe method using a Mitsubishi Chemical Analytech Loresta-GP with an ASP probe.Results and discussion
Metal source and alkanolamines
The most important advantage of CuF is its self-reduction ability, as it forms copper and releases hydrogen gas during the decomposition.27,28 Moreover, alkanolamines also show reduction abilities.9 This facilitates the reduction of Cu2+ from CuF to Cu(0), and prevents the oxidation of copper during sintering and reduces surface oxides from the commercially available fine copper particles. The low decomposition temperature of the CuF–alkanolamine complexes allows for the formation and sintering of copper particles to be performed under nitrogen gas only, thus negating the need for the use of reductive gases such as hydrogen or formic acid.24,26 At the same time, the low decomposition temperature meets the requirement of low temperature and cost for printed electronics, that is, printable on the plastic substrates with low glass transition temperature as well as on organic electronics parts. Finally, the decomposition of CuF results in H2 and CO2, without leaving any organic residues that can decrease the conductivity of the film. Usually, metallacycle complexes exhibit high stability. These complexes also exhibit low evaporation or decomposition temperatures.Stability of copper(II) formate–alkanolamine complexes
Alkanolamines including IPA, 2AB, and DEAE were selected to study the stability of MOD inks prepared using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of CuF and the alkanolamine. Fig. 2 shows the colour changes of different CuF–alkanolamine complexes over time. For the CuF–IPA and CuF–2AB complexes, there were no obvious colour changes after 14 days and 6 h, respectively. However, some solid components formed with the Cu–2AB complex after 4 h, as shown in Fig. 2b. In contrast, the colour of the CuF–DEAE complex began to change from indigo to light blue after 1 h (Fig. 2c). The colour of the CuF–DEAE complex continuously changed to light turbid blue with time. This indicates the formation of solid materials with light blue colour in the liquid.
1 molar ratio of CuF and the alkanolamine. Fig. 2 shows the colour changes of different CuF–alkanolamine complexes over time. For the CuF–IPA and CuF–2AB complexes, there were no obvious colour changes after 14 days and 6 h, respectively. However, some solid components formed with the Cu–2AB complex after 4 h, as shown in Fig. 2b. In contrast, the colour of the CuF–DEAE complex began to change from indigo to light blue after 1 h (Fig. 2c). The colour of the CuF–DEAE complex continuously changed to light turbid blue with time. This indicates the formation of solid materials with light blue colour in the liquid.
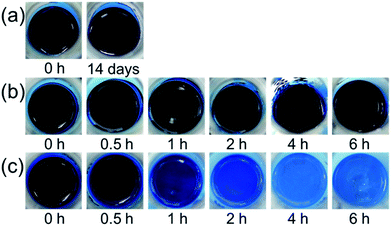 | ||
Fig. 2 Photographs of different CuF–alkanolamines complexes after various time: (a) CuF–IPA, (b) CuF–2AB, and (c) CuF–DEAE complexes (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios). 1 molar ratios). | ||
XRD measurements of the CuF–alkanolamine complexes (Fig. 3) were performed. After preparation, all the CuF–alkanolamine complexes exhibited an amorphous phase as noted by the XRD patterns. With a storage time of up to 14 days, negligible changes were observed in the XRD pattern of the CuF–IPA complex. The XRD patterns of the CuF–2AB complex showed intense peaks after keeping for 4 h, indicating the formation of crystalline products. Unfortunately, the solid products could not be identified. On the other hand, after only 0.5 h, the XRD pattern of the CuF–DEAE complex revealed peaks related to crystalline phases. By comparing this pattern with JCPDS cards, we found that copper formate hydroxide (CH2CuO3, Cu(HCOO)(OH)) was the main component of the corresponding solid (Fig. S2†).29 This compound is greenish blue, and the colour change observed in Fig. 2c can be explained by this compound.
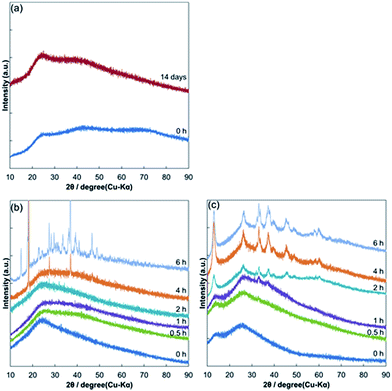 | ||
Fig. 3 X-ray diffraction patterns of different CuF–alkanolamine complexes over time: (a) CuF–IPA, (b) CuF–2AB, and (c) CuF–DEAE complexes (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios). 1 molar ratios). | ||
In both cases, the XRD peaks became sharper and more defined with increased storage times. Based on these results, it was concluded that among the alkanolamines used in this study, IPA formed the most stable complex with CuF.
The aforementioned results were attributed to the stability of the structures of different CuF–alkanolamine complexes (Fig. 1). According to hard soft acid base theory,30 IPA and 2AB can form bidentate interactions with copper, resulting in five-membered metallacycles with stable structures. However, as DEAE is a tertiary amine with two ethyl groups that bind to N, it is sterically hindered. Therefore, the bidentate structure shown in Fig. 1c should be more unstable than those of the other two complexes. Therefore, Cu(HCOO)(OH) was generated and formed a solid powder.
IPA and 2AB have similar molecular structures (Fig. 1a and b). However, CuF–IPA did not form any solid compounds, probably due to differences in the self-assembly of the two complexes. CuF–2AB complexes may stack on top of each other owing to their amphiphilic structure. Therefore, no colour change was observed in Fig. 2b, but self-assembled solid components were formed after several hours. We chose IPA as the optimal ligand for complex formation with CuF in the next step of our study due to the best stability of the CuF–IPA complex.
Conductivity of copper film prepared from CuF–IPA complex
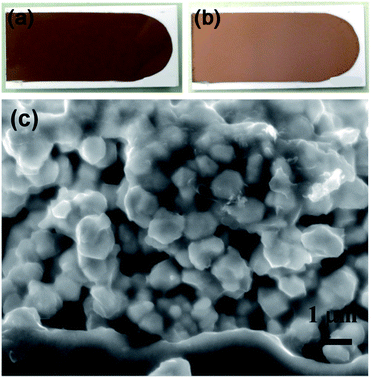
In order to investigate the resistivity of the copper film fabricated using the CuF–IPA complex, the printed complex was sintered under N2 at 100 °C. Layers of CuF–IPA complex were prepared using a doctor blade with a thickness of 100 μm.The blue layer of the CuF–IPA complex turned into a brown film (Fig. 4a and b) after 1 h of sintering at 100 °C under nitrogen flow (99.99%). In the XRD pattern of the sintered sample, all of the observed peaks belonged to metallic copper. This XRD pattern clearly indicates that reduction of Cu2+ to Cu(0) proceeds at 100 °C in the presence of IPA. The formation of metallic copper was due to the decomposition of CuF accompanied by the generation of H2 reductive gas. After reduction of Cu2+, IPA evaporates slowly because the boiling point of IPA is 160 °C. The decomposition of the CuF–IPA complex was also confirmed using TGA-DTA (Fig. S3†). Decomposition of CuF–IPA occurs even at temperatures lower than 100 °C. It is possible that copper ions were also reduced during heat treatment of CuF–IPA.22
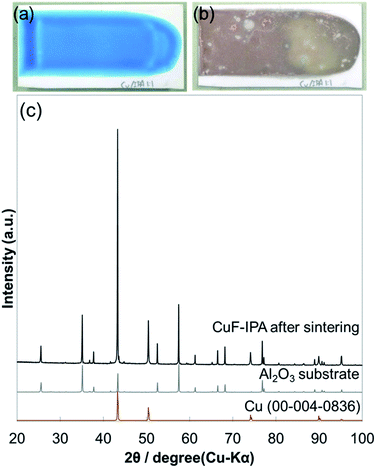 | ||
Fig. 4 (a–b) Photographs of CuF–IPA complex prepared using CuF and IPA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) before and after sintering at 100 °C under N2 for 1 h, respectively; (c) XRD pattern of the film shown in (b). 1 molar ratio) before and after sintering at 100 °C under N2 for 1 h, respectively; (c) XRD pattern of the film shown in (b). | ||
However, when only the CuF–IPA complex was used for printing, it was difficult to obtain a uniform copper film after sintering (Fig. 4a–b). Conductivity was not detected for the copper film obtained after sintering the CuF–IPA ink at 100 °C, owing to the formation of many cracks and the inhomogeneity of the copper film. This problem was also reported by Shin et al. when 2-amino-2-methyl-1-propanol was used as the ligand and isopropyl alcohol was used as the solvent to prepare a copper complex ink.22
Copper films prepared from CuF–IPA–Cu inks
To overcome the aforementioned problems, instead of using organic additives or sintering helpers as in previous studies,22–26 we introduced copper particles into the CuF–IPA complex to make the ink. The total copper concentration in this ink is approximately 65 wt%. In this way, the use of organic additives and sintering helpers as well as a reducing gas could be avoided while the packing density of the copper film was improved after the decomposition of the copper complex followed by the co-sintering of the added copper and generated copper particles. This can lead to a higher conductivity of the resulting copper film at a lower sintering temperature.The sintering temperature was reduced to 100 °C and the resistivity of the film was measured using the sample prepared by adding 0.8 μm-copper particles. A particle size of 0.8 μm was optimal in this study because in smaller particles, many protective agents, which cover the copper particles, prevent interactions between the copper particles during sintering. Conversely, bigger particles lead to a poor packing density owing to the large pore volume.
Fig. 5c shows the SEM image of the sintered copper films obtained from the CuF–IPA–Cu ink. The connections among copper particles were observed in the films prepared using 0.8 μm-copper particles after sintering for 1 h at 100 °C. Under these conditions, a resistivity of 9.0 × 10−6 Ω m was obtained. The relatively lower concentration of IPA contributed to the higher packing density of the obtained copper film. Fig. 5a and b show the photographs of the CuF–IPA–Cu ink after printing at room temperature and after sintering at 100 °C, respectively. Compared to the sintered film from the CuF–IPA complex, the sintered copper film prepared from the CuF–IPA–Cu ink exhibited much higher uniformity. No cracks were observed on the surface of the film. By adding copper particles into the ink, the packing density was significantly improved after sintering at 100 °C. Tight connections among copper particles were observed in the SEM image (Fig. 5c) of the film after sintering, which resulted in the low resistivity. This result demonstrated the effectiveness of the developed method in terms of producing a film with high conductivity at a low sintering temperature.
Conclusions
In conclusion, highly conductive copper films were achieved at a very low sintering temperature of 100 °C by using a copper-based MOD ink and without reducing gas for the first time. The avoidance of reducing gases, such as hydrogen, makes the system safer and simpler. The addition of copper particles to CuF–IPA complexes at a sintering temperature of 100 °C resulted in a film with a resistivity of 9.0 × 10−6 Ω m. The developed sintering process had significant advantages in the production of a highly conductive film for printing, including: (i) enhanced packing density, smoothness and uniformity of the copper film based on the direct sintering of the added copper particles and copper particles generated during the decomposition of the copper complex, (ii) avoided the use of organic additives and sintering helpers as well as reducing gas, and (iii) reduced energy and time. Sintering at 100 °C enabled us to use this system with PET films. Optimization of detailed sintering parameters and preparation of stable pastes or inks are underway in our laboratory and will be reported in due course.Acknowledgements
This work is partially supported by Hokkaido University. MTN acknowledges the financial support from the F3 program of Hokkaido University. The authors thank Dowa for providing us with copper fine particles.References
- M.-C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293 CrossRef CAS PubMed.
- J. N. Freitas, A. S. Gonçalves and A. F. Nogueira, Nanoscale, 2014, 6, 6371 RSC.
- P. Peng, A. Hu, A. P. Gerlich, G. Zou, L. Liu and Y. N. Zhou, ACS Appl. Mater. Interfaces, 2015, 7, 12597 CAS.
- N. Toshima and T. Yonezawa, New J. Chem., 1998, 22, 1179 RSC.
- T. Yonezawa, Kobunshi Ronbunshu, 2013, 70, 684 CrossRef CAS.
- T. Yonezawa, Y. Uchida and H. Tsukamoto, Phys. Chem. Chem. Phys., 2015, 17, 32511 RSC.
- Y. Lee, J. R. Choi, K. J. Lee, N. E. Stott and D. Kim, Nanotechnology, 2008, 19, 415604 CrossRef PubMed.
- D. Deng, Y. Cheng, Y. Jin, T. Qi and F. Xiao, J. Mater. Chem., 2012, 22, 23989 RSC.
- Y. Hokita, M. Kanzaki, T. Sugiyama, R. Arakawa and H. Kawasaki, ACS Appl. Mater. Interfaces, 2015, 7, 19382 CAS.
- K. V. Abhinav, R. V. Krishna Rao, P. S. Karthik and S. P. Singh, RSC Adv., 2015, 5, 63985 RSC.
- S. Magdassi, M. Grouchko and A. Kamyshny, Materials, 2010, 3, 4626 CrossRef CAS.
- A. Yabuki and N. Arriffin, Thin Solid Films, 2010, 518, 7033 CrossRef CAS.
- K. Ida, M. Tomonari, Y. Sugiyama, Y. Chujyo, T. Tokunaga, T. Yonezawa, K. Kuroda and K. Sasaki, Thin Solid Films, 2012, 520, 2789 CrossRef CAS.
- Y. Yong, T. Yonezawa, M. Matsubara and H. Tsukamoto, J. Mater. Chem. C, 2015, 3, 5890 RSC.
- M. Matsubara, T. Yonezawa and H. Tsukamoto, Bull. Chem. Soc. Jpn., 2015, 88, 1755 CrossRef.
- T. Yonezawa, H. Tsukamoto and M. Matsubara, RSC Adv., 2015, 5, 61290 RSC.
- C. Kim, G. Lee, C. Rhee and M. Lee, Nanoscale, 2015, 7, 6627 RSC.
- B. Lee, Y. Kim, S. Yang, I. Jeong and J. Moon, Curr. Appl. Phys., 2009, 9, e157 CrossRef.
- M. Matsubara, T. Yonezawa, T. Minoshima, H. Tsukamoto, Y. Yong, Y. Ishida, M. T. Nguyen, H. Tanaka, K. Okamoto and T. Osaka, RSC Adv., 2015, 5, 102904 RSC.
- J. Lee, B. Lee, S. Jeong, Y. Kim and M. Lee, Thin Solid Films, 2014, 564, 264 CrossRef CAS.
- G. Qin, A. Watanabe, H. Tsukamoto and T. Yonezawa, Jpn. J. Appl. Phys., 2014, 53, 096501 CrossRef.
- D.-H. Shin, S. Woo, H. Yem, M. Cha, S. Cho, M. Kang, S. Jeong, Y. Kim, K. Kang and Y. Piao, ACS Appl. Mater. Interfaces, 2014, 6, 3312 CAS.
- Y. Farraj, M. Grouchko and S. Magdassi, Chem. Commun., 2015, 51, 1587 RSC.
- Y.-H. Choi and S.-H. Hong, Langmuir, 2015, 31, 8101 CrossRef CAS PubMed.
- A. Yabuki and S. Tanaka, Mater. Res. Bull., 2012, 47, 4107 CrossRef CAS.
- S. J. Kim, J. Lee, Y.-H. Choi, D.-H. Yeon and Y. Byun, Thin Solid Films, 2012, 520, 2731 CrossRef CAS.
- A. K. Galwey, D. Jamieson and M. E. Brown, J. Phys. Chem., 1974, 78, 2664 CrossRef CAS.
- B. Lee, S. Jeong, Y. Kim, I. Jeong, K. Woo and J. Moon, Met. Mater. Int., 2012, 18, 493 CrossRef CAS.
- V. Krasilnikov, V. Antsygna and G. Bazuev, Russ. J. Inorg. Chem., 1995, 40, 1025 Search PubMed . JCPDS No. 00-050-0663.
- G. A. Lawrence, Introduction to Coordination Chemistry, J. Wiley & Sons, West Sussex, 2010 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: SEM image of the copper particles used for the CuF–IPA–Cu ink. XRD patterns of solid compounds generated from CuF–DEAE, and TGA-DTA curves of CuF–IPA complex. See DOI: 10.1039/c5ra25058g |
| ‡ Actual address: Department of Materials and Environment Engineering, National Institute of Technology, Sendai College, 48 Nodayama, Medeshima-Shiote, Natori-shi, Miyagi 981-1239, Japan. |
| This journal is © The Royal Society of Chemistry 2016 |