Preparation of a magnetic responsive immobilized lipase–cellulose microgel catalyst system: role of the surface properties of the magnetic cellulose microgel†
Abstract
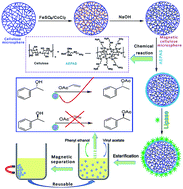
Surface modification of the magnetic cellulose particles has been conducted by using AEAPS, the modified magnetic cellulose particles were then used for the immobilization of lipase for catalysis reaction. The highest specific activity for AEAPS modified magnetic cellulose scaffold was increased to about 30 fold that of the free lipase without support. Furthermore, the reusability of the immobilized lipase on AEAPS modified magnetic cellulose particles was also improved, the relative activity of the ARCM-2 and AMRCM1-2 after being reused for 5 times still remained 72.8% and 71.2%, respectively. Moreover, the immobilized lipase on AEAPS modified magnetic cellulose particles was easily recovered without significant decrease in the specific activity. The immobilization of lipase on AEAPS modified magnetic cellulose particles led to the improvement in stability of activity and reusability for non-aqueous transesterification.

 Please wait while we load your content...
Please wait while we load your content...