Complexing agent study for environmentally friendly silver electrodeposition(ii): electrochemical behavior of silver complex
Abstract
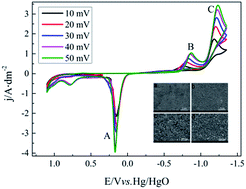
Electrochemical behaviors of a silver complex in an environmentally friendly silver plating bath with 5,5-dimethylhydantoin (DMH) and nicotinic acid (NA) as complexing agents were investigated in this paper. Like the cyanide based silver electroplating bath, silver deposits with smooth and compact morphologies, as well as high purity could be obtained from the studied DMH and NA based silver electroplating bath. The electrochemical behaviors of the silver complex in this cyanide-free silver electroplating were studied by cyclic voltammetry with different sweep rates to investigate the discharge process of the silver plating bath, the transfer coefficients and diffusion coefficient of the silver complex in this bath. The results of the chronoamperometry on a glass carbon electrode indicate that the nucleation processes of silver from the bath is a three-dimensional progressive nucleation process, and the growth of silver nucleation and the nucleation rate of silver deposit in the DMH and NA based silver plating bath were highly dependent on the applied potential.

 Please wait while we load your content...
Please wait while we load your content...