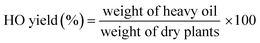Bio-oil production from eight selected green landscaping wastes through hydrothermal liquefaction†
Leichang Cao,
Gang Luo,
Shicheng Zhang* and
Jianmin Chen
Shanghai Key Laboratory of Atmospheric Particle Pollution and Prevention (LAP3), Department of Environmental Science and Engineering, Fudan University, Shanghai 200433, China. E-mail: zhangsc@fudan.edu.cn; Fax: +86-02165642297; Tel: +86-02165642297
First published on 29th January 2016
Abstract
This study investigated the potential of eight types of green landscaping waste as feedstock to produce bio-oil through hydrothermal liquefaction (HTL). The eight selected plants differed in terms of botanical classification, morphology, leaf state, and growth habit. Leaves and branches as waste from these plants were separately subjected to HTL in a high-pressure batch reactor at 300 °C for 0.5 h. Results indicated the bio-oils and biochars of leaves obviously differed from those of branches in terms of yields and higher heating values (HHVs). However, less difference in yields and HHVs was found for HTL products within the eight leaves even though they were different in composition components such as cellulose, hemicelluloses, and lignin. The same was observed for branches. The average bio-oil yields of the leaves and branches were 33.74 and 43.22 wt%, respectively. The optimal bio-oil yield was 50.44 wt%, which was obtained when Cinnamomum camphora branches were used as feedstock. The average HHVs of light and heavy oils in the leaves were 25.13 and 31.27 MJ kg−1, respectively. These HHVs were higher than those of light and heavy oils in the branches (21.51 and 28.71 MJ kg−1, respectively). Among the oil products, the heavy oil derived from Salix alba leaves yielded the optimum HHV (35.63 MJ kg−1). The mean HHV of biochar was 24.17 MJ kg−1, which was considerably higher than that of feedstock (17.21 MJ kg−1). Gas chromatography-mass spectrometry and Fourier-transform infrared spectrometry revealed the presence of value-added chemicals, such as phenolics, ketones, esters, acids, and alcohols, in bio-oils. The amounts of alkanes, alkenes, and alkynes in the bio-oils derived from the leaves were higher than those in the bio-oils derived from the branches. These results indicated the feasibility of using different types of green landscaping waste as feedstock to produce bio-oils with high HHV and yield through HTL.
1. Introduction
Current waste disposal methods, particularly of municipal and livestock waste, have caused serious problems because disposal methods can induce pollution and exacerbate waste production. Green waste is an important component of municipal waste. By the end of 2014, the urban landscape area of China was more than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 907
907![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 ha; this finding corresponds to a green coverage rate of 39.69% and a per capita green area of 12.64 m2. The annual green waste production in China is more than 250
500 ha; this finding corresponds to a green coverage rate of 39.69% and a per capita green area of 12.64 m2. The annual green waste production in China is more than 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons, and approximately 10% of this amount is recycled.1 With the continuous development of ecological construction and urban greening, green waste will considerably increase because of the lack of reasonable recycling. Most green waste is disposed with household waste; thus, the ecological cycle in soil is disrupted; as a result, soil fertility declines and environmental pollution issues ensue.2–4 As a type of biomass feedstock with high carbon content and relatively low harmful components, green wastes have attracted numerous studies regarding its utilization. The use of landfill and incineration are among the traditional treatments for green wastes.1 Although landfill operation is simple, extensive land resources are necessary and the transport of green wastes is inconvenient. Landfill leachate, stench, and gas also harm the surrounding environment, resulting in secondary pollution. Incineration can reduce the volume of wastes but will cause air pollution because of improper technical means and other reasons.5,6 Several countries are currently implementing a ban on the burning of green wastes.1 Therefore, developing a new, cost-effective, and environment-friendly technology to utilize green wastes is important.
000 tons, and approximately 10% of this amount is recycled.1 With the continuous development of ecological construction and urban greening, green waste will considerably increase because of the lack of reasonable recycling. Most green waste is disposed with household waste; thus, the ecological cycle in soil is disrupted; as a result, soil fertility declines and environmental pollution issues ensue.2–4 As a type of biomass feedstock with high carbon content and relatively low harmful components, green wastes have attracted numerous studies regarding its utilization. The use of landfill and incineration are among the traditional treatments for green wastes.1 Although landfill operation is simple, extensive land resources are necessary and the transport of green wastes is inconvenient. Landfill leachate, stench, and gas also harm the surrounding environment, resulting in secondary pollution. Incineration can reduce the volume of wastes but will cause air pollution because of improper technical means and other reasons.5,6 Several countries are currently implementing a ban on the burning of green wastes.1 Therefore, developing a new, cost-effective, and environment-friendly technology to utilize green wastes is important.
As a type of lignocellulosic biomass, green waste is primarily composed of cellulose, hemicellulose, and lignin; these organic compounds can be thermochemically converted into bio-fuels.7–12 Hydrothermal liquefaction (HTL) is a thermochemical processing technology and chemical reformulation process extensively investigated to produce bio-oil.13–15 In HTL, hot pressurized water or other solvents are used as a reaction medium and reactant. Biomass is depolymerized into bio-oil, biochar, gases, and water-soluble substances in an aerobic or anaerobic enclosure.15–19 Bio-oils can be used as fuels for burners, stationary diesel engines, turbines, or boilers. Bio-oils can also be upgraded or converted into transportation fuels, such as diesel and gasoline, and other products, including aromatics, polymers, asphalt, and lubricants.14,20
The HTL of lignocellulosic biomass has been extensively investigated because this process is reliable, available, and environmentally friendly.21–26 Minowa et al.27 successfully produced bio-oil from algae through HTL; as such, this process has been used as the basis of research on biomass liquefaction. Zhang et al.28 developed a method to prepare liquid fuel from desert shrub through HTL, which is suitable for all shrubs. Morup et al.29 initially liquefied the grains from a drying distiller; the liquid is soluble in bio-oil with a yield of 49.6%; thus, the scope of HTL has been further expanded. Wang et al.30 also successfully obtained a high bio-oil yield from an oilseed of Litsea cubeba through HTL at different temperatures (250–350 °C), times (30–120 min), reactor loadings (0.5–4.5 g), and Na2CO3 loadings (0–10%). Zhu et al.31 investigated the HTL of barley straw at temperatures ranging from 280 °C (subcritical water) to 400 °C (supercritical water). Zhu et al.31 found that the bio-oil yield increases slightly when the straw is liquefied at 280–320 °C. The bio-oil yield reaches a maximum of 34.9 wt% at 300 °C. Singh et al.32 examined the effect of the biomass/H2O ratio on the distribution of products in the HTL of water hyacinth. As the water hyacinth/H2O ratio increases from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 1
3 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, the total bio-oil yield increases from 6% to 16%; the yield further increases to 18% when the water hyacinth/H2O ratio increases to 1
6, the total bio-oil yield increases from 6% to 16%; the yield further increases to 18% when the water hyacinth/H2O ratio increases to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12. Liu et al.33 also investigated the effect of the cornstalk/H2O ratio on product yields during liquefaction. As the cornstalk/H2O ratio changes from 1
12. Liu et al.33 also investigated the effect of the cornstalk/H2O ratio on product yields during liquefaction. As the cornstalk/H2O ratio changes from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to 1
10 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, the bio-oil yield increases from 19.6% to 33.9%; when the cornstalk/H2O ratio further increases to 14%, the bio-oil yield decreases to 31.2%. This result is consistent with that observed by Yin et al.34
6, the bio-oil yield increases from 19.6% to 33.9%; when the cornstalk/H2O ratio further increases to 14%, the bio-oil yield decreases to 31.2%. This result is consistent with that observed by Yin et al.34
Although HTL of lignocellulosic biomass has been well studied, there are few literature reports on the potential of bio-oil production with different green waste through HTL, and little information is available regarding the difference of leaves and branches on the chemical composition and yield of bio-oil. Considering the diversity of the green landscaping plants, it is of considerable interest to select plants which are different in composition components such as cellulose, hemicelluloses, and lignin for HTL conversion processes, thereby investigating whether different composition components affect bio-products yield and quality. The findings will be useful for industrial bio-oil production from variety green wastes. The green landscaping plants used for bio-oil production in this study were Pinus sp. (PI), Cupressus funebris (CF), Platanus sp. (PL), Cinnamomum camphora (CC), Pittosporum tobira (PT), Distylium racemosum (DR), Viburnum odoratissinum (VO), and Salix alba (SA). Table S1 (ESI†) lists the information on these eight green landscaping plants. This study aimed to evaluate the feasibility of bio-oil production using different green wastes through HTL.
2. Experimental
2.1. Materials
All the green wastes were collected in the campus of Fudan University in March 2014. The plants samples were first dried in an oven (104 °C, 8 h) to get absolute dry feedstock. Afterwards, they were ground and screened into particles with a diameter of 40–60 mesh. The characteristics of the plants samples are given in Tables 1 and 2. Cellulose, hemicelluloses, and lignin content in the plants were analyzed following the protocol from the NREL Chemical Analysis and Testing Standard Procedures: NREL LAP, TP-510-42618.35–38 Before quantification, biomass samples underwent two-stage acid hydrolysis: (1) 72 wt% sulfuric acid for 1 h at 30 °C and (2) 4 wt% sulfuric acid for 1 h inside of an autoclave with the temperature held at 120 °C. Solid residues after the two-stage hydrolysis were deemed acid insoluble lignin (AIL). Saccharides, which were in the liquid phase after hydrolysis, were quantified using a HPLC with a Bio-Rad Aminex HPX-87P column equipped with a refractive index detector. The acid soluble lignin (ASL) was quantified by measuring its absorbance at 320 nm in a UV-visible spectrophotometer. Ash content in biomass was determined by burning sample at 575 °C for 6 h inside of a muffle furnace. In addition, acetone–ethanol (v/v = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) co-solvent was used to extract the fat of green wastes inside a Soxhlet extractor.38 All the chemicals were obtained from Aladdin Reagent Co., Ltd. (Shanghai).
1) co-solvent was used to extract the fat of green wastes inside a Soxhlet extractor.38 All the chemicals were obtained from Aladdin Reagent Co., Ltd. (Shanghai).
| Leaves | Pinus | Cupressus funebris | Platanus | Cinnamomum camphora | Pittosporum tobira | Distylium racemosum | Viburnum odoratissinum | Salix babylonica | Mean value |
|---|---|---|---|---|---|---|---|---|---|
| a Calculated by difference.b Determined by ICP-AES. | |||||||||
| Components | |||||||||
| Cellulose | 22.9 | 24.8 | 22.7 | 23.5 | 18.2 | 23.7 | 8.3 | 23.2 | 20.9 |
| Hemicellulose | 15.0 | 16.9 | 16.1 | 12.9 | 17.6 | 16.3 | 17.5 | 17.3 | 16.2 |
| Lignin | 31.0 | 16.8 | 23.6 | 16.8 | 10.6 | 23.8 | 20.1 | 18.3 | 20.1 |
| Ash | 1.3 | 7.4 | 6.0 | 8.0 | 10.7 | 10.0 | 9.8 | 8.8 | 7.7 |
| Ethanol extracts | 8.5 | 8.5 | 5.4 | 5.4 | 9.1 | 6.1 | 8.1 | 4.9 | 7.0 |
| Others | 21.4 | 25.6 | 26.3 | 33.5 | 33.8 | 20.1 | 36.2 | 27.6 | 28.1 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Ultimate | |||||||||
| C | 46.6 | 43.0 | 45.3 | 43.5 | 45.2 | 46.9 | 44.6 | 45.4 | 45.1 |
| H | 7.4 | 6.8 | 7.7 | 7.4 | 7.3 | 7.3 | 7.6 | 7.4 | 7.4 |
| Oa | 44.7 | 49.6 | 46.2 | 47.8 | 46.4 | 44.8 | 45.0 | 47.0 | 46.4 |
| N | 1.3 | 0.7 | 0.7 | 1.4 | 1.1 | 1.0 | 2.8 | 0.2 | 1.2 |
| Sb | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Atom ratios | |||||||||
| H/C | 1.9 | 1.9 | 2.0 | 2.1 | 1.9 | 1.9 | 2.0 | 2.0 | 2.0 |
| H/O | 2.7 | 2.2 | 2.7 | 2.5 | 2.5 | 2.6 | 2.7 | 2.5 | 2.5 |
| HHV (MJ kg−1) | 18.4 | 15.3 | 18.1 | 16.8 | 17.4 | 18.3 | 18.0 | 17.6 | 17.5 |
| Branches | Pinus | Cupressus funebris | Platanus | Cinnamomum camphora | Pittosporum tobira | Distylium racemosum | Viburnum odoratissinum | Salix babylonica | Mean value |
|---|---|---|---|---|---|---|---|---|---|
| a Calculated by difference.b Determined by ICP-AES. | |||||||||
| Components | |||||||||
| Cellulose | 26.9 | 23.0 | 27.7 | 27.8 | 26.7 | 24.5 | 25.4 | 25.9 | 26.0 |
| Hemicellulose | 20.8 | 19.0 | 19.9 | 18.4 | 21.2 | 16.4 | 18.9 | 17.0 | 18.9 |
| Lignin | 16.8 | 18.3 | 25.6 | 27.7 | 14.5 | 23.1 | 30.1 | 30.1 | 23.3 |
| Ash | 2.8 | 2.9 | 2.1 | 2.0 | 5.4 | 4.6 | 2.2 | 1.4 | 2.9 |
| Ethanol extracts | 7.0 | 5.9 | 2.6 | 4.9 | 5.6 | 4.5 | 3.4 | 3.8 | 4.7 |
| Others | 25.7 | 31.0 | 22.1 | 19.2 | 26.6 | 26.9 | 19.9 | 21.9 | 24.2 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Ultimate | |||||||||
| C | 45.8 | 43.7 | 45.6 | 46.1 | 46.1 | 47.4 | 44.1 | 46.2 | 45.6 |
| H | 7.2 | 6.8 | 7.0 | 6.9 | 7.0 | 6.9 | 6.7 | 7.3 | 7.0 |
| Oa | 46.7 | 49.3 | 47.2 | 46.9 | 46.8 | 45.6 | 49.1 | 46.1 | 47.2 |
| N | 0.3 | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 | 0.2 | 0.5 | 0.2 |
| Sb | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Atom ratios | |||||||||
| H/C | 1.9 | 1.9 | 1.8 | 1.8 | 1.8 | 1.7 | 1.8 | 1.9 | 1.8 |
| H/O | 2.5 | 2.2 | 2.4 | 2.3 | 2.4 | 2.4 | 2.2 | 2.5 | 2.4 |
| HHV (MJ kg−1) | 17.5 | 15.6 | 17.0 | 17.0 | 17.3 | 17.8 | 15.7 | 17.8 | 17.0 |
2.2. HTL and separation procedures
The hydrothermal liquefaction experiments were conducted in a 250 mL GSH-0.25 type autoclave with a temperature probe in it (Fig. 1). The autoclave was heated with an electrical furnace (2 kW, 10 °C min−1). In a typical experiment, 15 g (dry basis) of plant sample and 150 mL water was loaded into the reactor. An internal magnetic stirrer agitated the reactants at a frequency of 180 rpm. When the temperature reached 300 °C, it was controlled for 30 minutes (reaction time). Afterwards, the reactor was cooled down fast to the room temperature by cooling water system installed inside the reactor. The separating procedure for HTL products is shown in Fig. 2. After cooling procedure, the gas was collected for GC analysis. The other products were removed from the reactor by washing with 100 mL deionized water for five times. After vacuum filtration (G3 filtrator with a 0.22 μm membrane), the liquid and solid phases were separated. The bio-oil was extracted from two phases by ethyl acetate (EA). Given its moderate chemical polarity, which is similar to most components of crude bio-oil, EA is frequently used as a solvent to extract bio-oils during the thermal-chemical conversion of biomass.39–42 Two EA solutions were separated from the aqueous and solid phases by using a separatory funnel (aqueous phase) and a G3 filtrator (solid phase), respectively. Excess anhydrous sodium sulfate was used to remove water and residual glycerol in the solutions. Subsequently, both solutions were evaporated to remove EA; thus, two kinds of bio-oil were obtained: light oil (LO, from aqueous phase) and heavy oil (HO, from solid phase). The EA insoluble fractions were designated as water products (WP) and biochar (after drying). The yield of each fraction was calculated as follows:2.3. Elemental analysis and higher heating value (HHV) calculation
The carbon (C), hydrogen (H), and nitrogen (N) contents in the feedstocks, bio-oils, and biochars were determined using an elemental analyzer (VARIO EL III, Elementar Inc., Germany), while their oxygen (O) contents were calculated by difference. H and C indicated the HHV of the bio-oil. The molar ratio of H and C determined the saturation of the hydrocarbons in the bio-oil. To determine sulfur (S) contents in the feedstocks, bio-oils, and biochars, a preliminary digestion was performed with concentrated nitric acid in an oil-bath at 140 °C, and then with hydrogen peroxide (30%, v/v) until the digested solution became transparent. Then, the volume of digested solution was made up to 100 mL using deionized water. The S concentration in the digested solution was determined by inductively coupled plasma-atomic emission spectrometry (ICP-AES, Optima 7300 DV, Perkin-Elmer Co., Waltham, MA) and the S contents in the feedstocks, bio-oils, and biochars were calculated correspondingly.The data obtained from the ultimate analysis were also used to calculate the high heating value (HHV). Dulong formula (eqn (1)) was used to calculate the HHV because the oxygen content in the precursors and the products was found to be greater than 15%.4
| HHV (MJ kg−1) = 0.3383C + 1.442 × (H − O/8) | (1) |
2.4. Fourier transform infrared spectroscopy (FTIR)
FTIR was used to investigate the functional groups' compositions of the bio-oil, which was obtained by a spectrometer (SHIMADZU IR Prestige 21, 400 cm−1–4000 cm−1, resolution = 2 cm−1, potassium bromide disc technique).2.5. Gas chromatography-mass spectrometry (GC-MS) for the qualitative analysis of bio-oil
GC-MS analysis of HO was performed on Thermo FOCUS DSQ with a HP-5 ms column (5% phenyl and 95% dimethylpolysiloxane, 30 mm × 0.25 mm × 0.25 μm). The carrier gas was helium with a flow rate at 1 mL min−1. A total of 1 μL of THF solution of HO (0.15 g 10 mL−1 THF) was injected into the column. The GC oven temperature program was as follows: hold at 60 °C for 2 min, raise to 300 °C with a heating rate of 20 °C min−1 and hold for 5 min. GC-MS analysis of LO was carried out using Finnigan Voyager with a HP-INNOWax column (100% polyethylene glycol, 30 mm × 0.25 mm × 0.25 μm). The carrier gas was also helium with a flow rate of 1 mL min−1. A total of 1 μL ether solution of WSO (0.10 g 10 mL−1 ether) was injected into the column. The GC oven temperature program was as follows: hold at 60 °C for 2 min, raise to 250 °C with a heating rate of 15 °C min−1 and hold for 10 min. Identification of compounds was performed by analyzing the MS data using the HP ChemStation software (GC database NIST14).2.6. Gas analysis
The identification and quantification of gases were done by using a gas chromatography (GC)-thermal conductivity detector (GL Science Inc., GC323, column: active carbon 30–60 mesh size) using an absolute calibration curve method.In the present investigation, average values for a minimum of triplicate measurements were reported in the figures and tables with a relative standard deviation of <5% in all cases.
3. Results and discussion
3.1. Characterization of the waste samples
The mean cellulose, hemicellulose, and lignin contents in the leaves were lower than those in the branches (Tables 1 and 2). This trend agrees with the phytobiology of leaves and branches. Cellulose, hemicellulose, and lignin are found in a plant cell wall; their content varies among different parts of a plant. With their higher rate of lignification in the cell wall, branches normally contain more lignin and cellulose than leaves.43–45 However, the ash and extract content of the leaves are significantly higher than those of branches. These results suggest that branches demonstrate further potential to produce more bio-oils and chemicals because of their relatively lower ash content but higher cellulose and hemicellulose content than leaves. Among the major components of plants, lignin exhibits the most stable structure; in its structure, the molecular chains are difficult to fracture but are easily coked; therefore, lignin is the most difficult to liquefy. By contrast, the structure of cellulose and hemicellulose is relatively loose, with weak bonds and poor thermal stability; as such, cellulose and hemicellulose are easily degraded.46 For definitive analysis, the C content of leaves was kept equal to that of branches. The H content of leaves was higher than that of branches; the inverse was true with the O content. The significantly low N and S content should be considered because the conversion into NOx and SOx precursors is avoided during the hydrothermal process.25 The high H/C atomic ratio of leaves and branches shows their strong aliphatic property; this finding suggests the presence of long chains with CH2 groups.47 The HHV of biomass is a highly important factor in terms of waste-to-energy conversion.48,49 The HHVs of leaves and branches were all low (<18 MJ kg−1). Therefore, the direct use of green waste for fuel is inefficient; therefore, novel methods should be developed to process green wastes to produce value-added fuels and chemicals. Given that the green landscaping wastes are typical lignocellulosic biomass, the following HTL conditions were selected for the current study: 300 °C, 150 mL/15 g (water-to-feed ratio), and residence time of 30 min. Excellent reviews for the HTL conditions of biomass can be used as ref. 25, 26 and 50.3.2. Product yield
As shown in Fig. 3, the bio-oil yields from leaves were clearly lower than those from branches; this result agreed with the characteristics of leaves and branches. The light oil yield from leaves and branches was not significantly different. However, the yields of heavy oils from branches were clearly higher than those from leaves. The average yields of bio-oils derived from leaves and branches were 33.74% (from 25.21% to 40.89%) and 43.22% (from 39.41% to 50.44%), respectively. These figures are comparable to the HTL of biomass in conventional batch reactors.7,9,36 Among the feedstocks, C. camphora (CC) and D. racemosum (DR) exhibited excellent oil productivity. The biochar yields of both leaves and branches were approximately 20%.Various types of biomass are available worldwide. Lignocellulosic biomass is the most commonly used in HTL. The main compositions of lignocellulosic biomass are cellulose, hemicellulose, and lignin.51,52 Different biomass have different compositions, which can remarkably affect the results of hydrothermal reaction. Feng et al.53 investigated the effects of white pine bark, white spruce bark, and white birch bark on the bio-oil yield in HTL under an initial N2 pressure of 2.0 MPa at 300 °C for 15 min. The bio-oil yields of cellulose, hemicellulose, and lignin during liquefaction were 36%, 58%, and 67%, respectively, which demonstrated the influence of the biomass types on bio-oil yield. Karagoz et al.54 also investigated the HTL of several biomasses (cellulose, lignin, sawdust, and rice husk) in identical conditions (280 °C, 15 min); their group found that cellulose exhibited higher bio-oil conversion than lignin. These results can be attributed to several reasons. First, lignin is the most stable of the three basic compositions, and its molecular chain is difficult to fracture and liquefy but is easy to coke.55 Second, cellulose and hemicellulose have a simpler structure, weaker bonding force, and poorer stability than lignin; thus, they are more easily degraded.56 Demirbas et al.57 investigated the HTL of nine types of biomass with different lignin content, which were liquefied in an autoclave at non-catalytic runs. The linear functions of the bio-oil yield and residue char yield obtained from the non-catalytic aqueous liquefaction of the biomass samples in terms of lignin content (wt%) are as follows:
| Bio-oil yield (%) = 42.548 − 0.388 × lignin content | (2) |
| Biochar yield (%) = 1.979 + 0.868 × lignin content | (3) |
Consequently, the degradation of lignin was difficult, and lignin mostly appeared in residue fraction; in addition, the yield of bio-oil changed with the lignin content.58 The yield of bio-oil tended to decrease over time with the increasing lignin content. However, some researchers generated opposite conclusions.59,60 For instance, Chen et al.60 found that the bio-oil yields derived from palm kernel shells (lignin content: 33.5%), dietary fibers (lignin content: 30.6%), and pomace (lignin content: 18.6%) were 38.53%, 34.32%, and 37.39%, respectively.
In the present study, Fig. 3(a) and Table 1 show that the yields of bio-oils from leaves do not correspond to the holocellulose (cellulose + hemicelluloses) composition of leaves. Conversely, Fig. 3(b) and Table 2 show that the bio-oil yield from branches basically correspond to the holocellulose composition of feedstocks. For the biochars (Fig. 3), the yields of leaves primarily corresponded to the lignin composition of the leaves, but this trend was not true for branches. These results further indicate that the effects of cellulose, hemicellulose, and lignin content on the hydrothermal bio-oil and biochar yields are not absolute. The synergistic effect of the three components and the different feedstocks affected the yields of bio-oil and biochar.
In addition, some different features in their composition of green landscaping wastes may also affect the yields of bio-oil and biochar. As it can be seen in Fig. 3, Tables 1 and 2, the total content of cellulose, hemicellulose, and lignin for leaves and branches were all lower than 75%, which were basically lower than those of stem wood biomass (>80%).39,54,60 On the other hand, about 4.71–7.00% ethanol extracts and 24.17–28.06% water extracts (in “others”, Tables 1 and 2) were in the leaves and branches. These extracts contained terpenoid, pigment, fat, resin, pectin, protein, alkaloid etc. In view of their considerable amount and complicated chemical properties, the effect of these extracts on hydrothermal conversion of the feedstocks cannot be ignored. Therefore, the results in the present work indicate that apart from the synergistic effect of the three components and feedstock species, the extracts may also affect the yields of bio-oil and biochar in biomass hydrothermal conversion.
3.3. Application potential of biochar and gas products
The detailed elemental analysis and HHVs of biochars derived from leaves and branches are shown in Tables S2 and S3 (ESI†), respectively. Fig. S1 (ESI†) shows the HHV comparison between biochars and feedstocks. The mean HHVs of biochars were considerably higher than those of leaves and branches; this result was primarily caused by the significantly higher C content of biochars. The mean HHVs of biochars derived from leaves and branches were as high as 23.43 and 24.42 MJ kg−1, respectively. These biochar HHVs are comparable to those of soft coal (20.93–33.50 MJ kg−1) and coke (25.12–29.30 MJ kg−1) and are not significantly lower than that of standard coal (29.26 MJ kg−1).60 The N content of biochar derived from leaves and branches was 0.93% and 0.89% respectively; both values are clearly lower than those for several of the reported biochars, such as palm leaf rib (2.74%),4 Russian olive seeds (1.91%),19 and Jatropha curcas shells (1.11%).61 The considerably low N and S content of biochar were also favorable characteristics in solid fuels by avoiding the emission of NOx and SOx during burning.4,19 Therefore, biochar can be considered an additional value-added product of bio-oil production from green landscaping wastes.The mean yield of gas products derived from leaves and branches were 8.22% and 9.17%, respectively. As shown in Fig. S2 and Table S4 (ESI†), CO2, H2, CO, and CH4 were the main components of the gases from both leaves and branches. The amount of CO2 was much larger than other gases, which agreed with previous work.13,62,63 The larger CO2 content of the gas derived from branches indicated that the branches decomposed more fiercely than the leaves, thereby also verifying the high bio-oil yield from branches.
3.4. Characterization of bio-oils
| Bio-oils | Element & HHV | Pinus | Cupressus funebris | Platanus | Cinnamomum camphora | Pittosporum tobira | Distylium racemosum | Viburnum odoratissinum | Salix alba | Mean value |
|---|---|---|---|---|---|---|---|---|---|---|
| a Calculated by difference.b Determined by ICP-AES. | ||||||||||
| Light oil | C | 60.1 | 62.0 | 57.2 | 62.1 | 63.8 | 61.4 | 64.5 | 66.1 | 62.2 |
| H | 6.0 | 6.6 | 6.1 | 6.6 | 7.3 | 6.2 | 5.8 | 7.4 | 6.5 | |
| Oa | 32.7 | 29.8 | 35.9 | 29.0 | 25.9 | 31.6 | 27.5 | 21.2 | 29.2 | |
| N | 1.2 | 1.6 | 0.8 | 2.3 | 3.0 | 0.7 | 2.2 | 5.3 | 2.2 | |
| S | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | |
| H/C | 1.2 | 1.3 | 1.3 | 1.3 | 1.4 | 1.2 | 1.1 | 1.3 | 1.3 | |
| H/O | 2.9 | 3.5 | 2.7 | 3.7 | 4.5 | 3.2 | 3.4 | 5.6 | 3.7 | |
| HHV/(MJ kg−1) | 23.1 | 25.1 | 21.7 | 25.4 | 27.4 | 24.1 | 25.2 | 29.2 | 25.1 | |
| Heavy oil | C | 69.8 | 69.2 | 70.0 | 74.0 | 71.0 | 64.1 | 71.7 | 73.7 | 70.4 |
| H | 7.6 | 7.0 | 6.3 | 8.4 | 8.5 | 6.8 | 8.1 | 9.2 | 7.7 | |
| Oa | 21.6 | 23.2 | 23.0 | 16.1 | 19.0 | 28.4 | 19.0 | 14.1 | 20.6 | |
| N | 1.1 | 0.7 | 0.7 | 1.5 | 1.5 | 0.7 | 1.2 | 3.1 | 1.3 | |
| Sb | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| H/C | 1.3 | 1.2 | 1.1 | 1.4 | 1.4 | 1.3 | 1.4 | 1.5 | 1.3 | |
| H/O | 5.7 | 4.8 | 4.4 | 8.3 | 7.2 | 3.8 | 6.8 | 10.4 | 6.4 | |
| HHV/(MJ kg−1) | 30.7 | 29.3 | 28.6 | 34.2 | 32.9 | 26.3 | 32.5 | 35.6 | 31.3 | |
| Bio-oils | Element & HHV | Pinus | Cupressus funebris | Platanus | Cinnamomum camphora | Pittosporum tobira | Distylium racemosum | Viburnum odoratissinum | Salix alba | Mean value |
|---|---|---|---|---|---|---|---|---|---|---|
| a Calculated by difference.b Determined by ICP-AES. | ||||||||||
| Light oil | C | 57.5 | 56.5 | 57.8 | 56.8 | 57.4 | 58.5 | 58.2 | 57.6 | 57.5 |
| H | 6.0 | 5.9 | 5.4 | 5.8 | 6.0 | 6.0 | 6.4 | 6.2 | 6.0 | |
| Oa | 36.1 | 37.5 | 36.6 | 37.3 | 36.3 | 35.3 | 35.2 | 35.7 | 36.2 | |
| N | 0.3 | 0.2 | 0.3 | 0.2 | 0.3 | 0.2 | 0.3 | 0.5 | 0.3 | |
| S | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | |
| H/C | 1.3 | 1.3 | 1.1 | 1.2 | 1.3 | 1.2 | 1.3 | 1.3 | 1.2 | |
| H/O | 2.7 | 2.5 | 2.4 | 2.5 | 2.7 | 2.7 | 2.9 | 2.8 | 2.6 | |
| HHV/(MJ kg−1) | 21.6 | 20.8 | 20.7 | 20.8 | 21.6 | 22.1 | 22.5 | 22.1 | 21.5 | |
| Heavy oil | C | 70.2 | 69.1 | 66.1 | 69.7 | 68.5 | 71.0 | 68.5 | 69.6 | 69.1 |
| H | 6.9 | 6.4 | 6.3 | 6.7 | 6.1 | 7.5 | 6.4 | 7.1 | 6.7 | |
| Oa | 22.7 | 24.2 | 27.2 | 23.3 | 24.9 | 21.3 | 24.7 | 22.3 | 23.8 | |
| N | 0.3 | 0.3 | 0.3 | 0.3 | 0.4 | 0.2 | 0.4 | 1.0 | 0.4 | |
| Sb | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| H/C | 1.2 | 1.1 | 1.2 | 1.2 | 1.1 | 1.3 | 1.1 | 1.2 | 1.2 | |
| H/O | 4.8 | 4.2 | 3.7 | 4.6 | 3.9 | 5.7 | 4.1 | 5.1 | 4.5 | |
| HHV/(MJ kg−1) | 29.5 | 28.2 | 26.6 | 29.1 | 27.5 | 31.0 | 27.9 | 29.8 | 28.7 | |
The assignment of structural group of the obtained bio-oils is based on published FTIR spectra and standard spectra in the FTIR libraries (e.g., Aldrich Condensed Phase FTIR Library).39,61 The main absorption bands from light oils of both leaves and branches were relatively similar (Fig. 5 and 6). The FTIR spectrum of heavy oils is relatively different from that of light oils. The absorption bands at 2938, 2927, 2883, and 2874 cm−1 in heavy oils were clearly stronger than those in light oils, thereby confirming that aliphatic groups were more abundant in heavy oils. This result was consistent with the elemental analysis for the comparison between heavy and light oils; thus, C content was higher in heavy oils. The O–H stretching vibration between 3200 and 3400 cm−1 indicated the presence of phenols and alcohols in bio-oils. The strong and broad C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations between 1640 and 1820 cm−1 indicated the presence of esters, carboxylic acids, ketones, and aldehydes. The strongest and overlapping absorption bands between 940 and 1325 cm−1 verified the presence of significant amounts of phenols, acids, and esters in bio-oils.
O stretching vibrations between 1640 and 1820 cm−1 indicated the presence of esters, carboxylic acids, ketones, and aldehydes. The strongest and overlapping absorption bands between 940 and 1325 cm−1 verified the presence of significant amounts of phenols, acids, and esters in bio-oils.
For light oils, the absorption bands at 2938, 1236, and 813 cm−1 of leaves were stronger than those of branches. These findings indicated the presence of more aliphatic compounds, ethers, alcohols, and aromatic compounds in the light oils from leaves.
Compared with heavy oils from leaves, those from branches showed no absorption bands at 752 cm−1, thereby indicating the higher levels of aromatic compounds in the heavy oils from leaves. The light and heavy oils from branches exhibited an absorption band at 625 cm−1 compared with those of leaves, thereby clearly demonstrating the existence of aliphatic ketones, particularly in the heavy oils from branches.
The GC-MS data indicated the presence of value-added chemicals, such as phenolics, cyclic ketones, ketones, and alcohols in light oils from both leaves and branches. Phenolics, aliphatics, esters, acids, ketones, alcohols, and furans were also the main products in heavy oils derived from both leaves and branches. These results agree with the elemental and FTIR analyses; thus, relatively more aliphatics in heavy oils made the C contents higher than those of light oils. Phenolics are the main products in both light and heavy oils. The relative contents of esters and acids in heavy oils were considerably higher than those in light oils. Cyclopentenones can be considered as additional main products of bio-oils. The content of cyclopentenones in heavy oils from Cupressus funebris branch was >30%. Greater amounts of alkane, alkene, and alkyne were found in bio-oils derived from leaves than in those derived from branches. This result also agrees with the elemental and HHV analyses; thus, bio-oils yielded enhanced C and H contents and increased HHVs.
Aliphatics are important compounds when bio-oils are used as fuels. The aliphatics in heavy oils from leaves had a mean RPA of 44.6%. The total RPA of aliphatics in C. funebris was as high as 48.8%.
No amine compounds were detected except in the heavy oil from S. alba leaf, which agrees well with the elemental analysis. The N content of heavy oil from S. alba leaf was clearly higher than those of the other oils.
One of the advantages of bio-oil fuels is its low sulfur content compared with other fossil fuels.4,39 In this study, organosulfur species were not observed in all the feedstocks, which is consistent with the insignificant amount of sulfur in the corresponding raw materials.
4. Conclusions
This study demonstrates the high-yield production of bio-oils from eight selected green landscaping wastes. Although the cellulose, hemicellulose, and lignin contents of the selected green wastes were distinct, the yields and HHVs of the derived bio-oils were slightly different. This difference indicated that the industrial bio-oil production from various green wastes is relatively convenient because classification is not necessary. The average bio-oil yields of leaves and branches were 33.74 wt% and 43.22 wt%, respectively. The optimal bio-oil yield was 50.44 wt%, which was achieved when C. camphora branch was used as feedstock. The average HHVs of light oil and heavy oil of leaves were 25.13 and 31.27 MJ kg−1, respectively, which were higher than those of branches (21.51 and 28.71 MJ kg−1, respectively). Among the oil products, the heavy oil derived from S. alba leaf produced the optimum HHV (35.63 MJ kg−1). The prepared bio-oils contained very low amounts of N and S. In addition, the mean HHV of biochar was 24.17 MJ kg−1; thus, biochar can be regarded as an additional product (fuel or carbon materials) of bio-oil production. GC-MS data revealed the presence of value-added chemicals, such as phenolics, cyclic ketones, ketones, and alcohols, in the light oils from leaves and branches. Phenolics, esters, acids, ketones, alcohols, and furans were the main products in the heavy oils derived from leaves and branches. In addition, as compared with branches, much more alkane, alkene and alkyne were found in bio-oil derived from leaves. FTIR data indicated more functional groups in carboxylic acid, ketone, aldehydes and esters in heavy oil as compared with light oil. Moreover, more aliphatic structures were found in bio-oils from leaves. The results indicated that it is feasible to use green landscaping waste as feedstock for producing bio-oil and bio-based chemicals.Acknowledgements
The authors are thankful for the financial support from the Walt Disney Company (Shanghai) Limited, the National Natural Science Foundation of China (No.583 21407027), the National Key Technology Support Program (No., 2015, BAD15B06), and the Shanghai Talent Development Fund (No., 2014, 14). The authors are also grateful for the provision of a scholarship to Leichang Cao by Shanghai Tongji Gao Tingyao Environmental Science & Technology Development Foundation. Lastly, the authors thank anonymous reviewers for fruitful suggestions.References
- D. Wu, Z. T. Huang, K. Yang, D. Graham and B. Xie, Environ. Sci. Technol., 2015, 49, 4122–4128 CrossRef CAS PubMed.
- S. T. Tan, H. Hashim, J. S. Lim, W. S. Ho, C. T. Lee and J. Yan, Appl. Energy, 2014, 136, 797–804 CrossRef CAS.
- M. Elsamadony, A. Tawfik and M. Suzuki, Appl. Energy, 2015, 149, 272–282 CrossRef CAS.
- F. Abnisa, A. Arami-Niya, W. M. A. W. Daud, J. N. Sahu and I. M. Noor, Energy Convers. Manage., 2013, 76, 1073–1082 CrossRef CAS.
- N. Scarlat, V. Motola, J. F. Dallemand, F. Monforti-Ferrario and L. Mofor, Renewable Sustainable Energy Rev., 2015, 50, 1269–1286 CrossRef.
- H. M. Z. Hossain, Q. H. Hossain, M. M. U. Monir and M. T. Ahmed, Renewable Sustainable Energy Rev., 2014, 39, 35–41 CrossRef.
- J. Zheng, M. Zhu and H. Wu, Waste Manag., 2015, 43, 230–238 CrossRef CAS PubMed.
- Y. H. Chan, S. Yusup, A. T. Quitain, Y. Uemura and M. Sasaki, J. Supercrit. Fluids, 2014, 95, 407–412 CrossRef CAS.
- R. Kaushik, G. K. Parshetti, Z. Liu and R. Balasubramanian, Bioresour. Technol., 2014, 168, 267–274 CrossRef CAS PubMed.
- W. Chen, Bioresour. Technol., 2014, 152, 130–139 CrossRef CAS PubMed.
- M. He, Fuel, 2012, 102, 506–513 CrossRef CAS.
- S. Kumagai, R. Matsuno, G. Grause, T. Kameda and T. Yoshioka, Bioresour. Technol., 2015, 178, 76–82 CrossRef CAS PubMed.
- R. Tungal and R. V. Shende, Appl. Energy, 2014, 134, 401–412 CrossRef CAS.
- J. H. Guo, R. X. Ruan and Y. Zhang, Ind. Eng. Chem. Res., 2012, 51, 6599–6604 CrossRef CAS.
- J. Gan, W. Q. Yuan, L. Johnson, D. H. Wang, R. Nelson and K. Zhang, Bioresour. Technol., 2012, 116, 413–420 CrossRef CAS PubMed.
- D. R. Vardon, ACS Sustainable Chem. Eng., 2013, 1, 1286–1294 CrossRef CAS.
- W. Shi, S. Li, J. F. Jia and Y. P. Zhao, Ind. Eng. Chem. Res., 2013, 52, 586–593 CrossRef CAS.
- D. C. Elliott, G. G. Neuenschwander and T. R. Hart, ACS Sustainable Chem. Eng., 2013, 1, 389–392 CrossRef CAS.
- K. Tekin, Energy Fuels, 2015, 27, 4382–4392 CrossRef.
- E. A. Ramos-Tercero, A. Bertucco and D. W. F. Brilman, Energy Fuels, 2015, 29, 2422–2430 CrossRef CAS.
- J. D. Jia, K. T. Sun and D. P. Jiang, J. Agric. Sci. Technol., 2014, 16, 1–6 Search PubMed.
- P. T. Patil, U. Armbruster and A. Martin, J. Supercrit. Fluids, 2014, 93, 121–129 CrossRef CAS.
- D. C. Elliott, P. Biller, A. B. Ross, A. J. Schmidt and S. B. Jones, Bioresour. Technol., 2015, 178, 147–156 CrossRef CAS PubMed.
- B. E. Hartman and P. G. Hatcher, Fuel, 2015, 156, 225–233 CrossRef CAS.
- J. A. Ramirez, R. J. Brown and T. J. Rainey, Energies, 2015, 8, 6765–6794 CrossRef CAS.
- Y. Guo, T. Yeh, W. Song, D. Xu and S. Wang, Renewable Sustainable Energy Rev., 2015, 48, 776–790 CrossRef CAS.
- T. Minowa, F. Zhen and T. Ogi, J. Supercrit. Fluids, 1998, 13, 253–259 CrossRef CAS.
- B. Zhang, M. Keitz and K. Valentas, J. Anal. Appl. Pyrolysis, 2009, 84, 18–24 CrossRef CAS.
- A. Kruse, A. Funke and M. Titirici, Curr. Opin. Chem. Biol., 2013, 17, 515–521 CrossRef CAS PubMed.
- F. Wang, Z. Chang, P. Duan, W. Yan, Y. Xu, L. Zhang, J. Miao and Y. Fan, Bioresour. Technol., 2013, 149, 509–515 CrossRef CAS PubMed.
- Z. Zhu, L. Rosendahl, S. S. Toor, D. Yu and G. Chen, Appl. Energy, 2015, 137, 183–192 CrossRef CAS.
- R. Singh, B. Balagurumurthy, A. Prakash and T. Bhaskar, Bioresour. Technol., 2015, 178, 157–165 CrossRef CAS PubMed.
- H. M. Liu, M. F. Li and R. C. Sun, Bioresour. Technol., 2013, 128, 58–64 CrossRef CAS PubMed.
- S. D. Yin, D. Ryan, M. Harris and Z. Tan, Bioresour. Technol., 2010, 101, 3657–3664 CrossRef CAS PubMed.
- P. K. Rout, M. K. Naik, S. N. Naik, V. V. Goud, L. M. Das and A. K. Dalai, Energy Fuels, 2009, 23, 6181–6188 CrossRef CAS.
- H. Liu, F. Wang and Y. Liu, J. Anal. Appl. Pyrolysis, 2014, 108, 136–142 CrossRef CAS.
- C. Miao, M. Chakraborty and S. Chen, Bioresour. Technol., 2012, 110, 617–627 CrossRef CAS PubMed.
- M. Chakraborty, C. Miao, A. McDonald and S. Chen, Fuel, 2012, 95, 63–70 CrossRef CAS.
- J. P. Cao, et al., Bioresour. Technol., 2011, 102, 2009–2015 CrossRef CAS PubMed.
- D. Zhou, L. Zhang, S. C. Zhang, H. B. Fu and J. M. Chen, Energy Fuels, 2010, 24, 4054–4061 CrossRef CAS.
- D. Zhou, S. C. Zhang, H. Fu and J. M. Chen, Energy Fuels, 2012, 26, 2342–2351 CrossRef CAS.
- A. Rahimi, A. Ulbrich, J. J. Coon and S. S. Stah, Nature, 2014, 515, 249–252 CrossRef CAS PubMed.
- Y. Wang, M. Chantreau, R. Sibout and S. Hawkins, Front. Plant Sci., 2013, 4, 1–14 Search PubMed.
- J. Barrosy, H. Serk, I. Granlundz and E. Pesquet, Ann. Bot., 2015, 115, 1503–1574 Search PubMed.
- J. H. Grabber, J. Ralph and R. D. Hatfield, J. Agric. Food Chem., 2002, 50, 6008–6016 CrossRef CAS PubMed.
- S. Feng, Fuel, 2014, 116, 214–220 CrossRef CAS.
- W. J. Liu, K. Tian, H. Jiang, X. S. Zhang, H. S. Ding and H. Q. Yu, Environ. Sci. Technol., 2012, 46, 7849–7856 CrossRef CAS PubMed.
- X. Hu, Bioresour. Technol., 2012, 123, 249–255 CrossRef CAS PubMed.
- J. F. Stanzione, P. A. Giangiulio, J. M. Sadler, J. J. L. Scala and R. P. Wool, ACS Sustainable Chem. Eng., 2013, 1, 419–426 CrossRef CAS.
- J. Akhtar and N. A. S. Amin, Renewable Sustainable Energy Rev., 2011, 15, 1615–1624 CrossRef CAS.
- A. A. Peterson, F. Vogel, R. P. Lachance, M. Froling, J. M. J. Antal and J. W. Tester, Energy Environ. Sci., 2008, 1(1), 32–65 CAS.
- E. Panisko, T. Wietsma, T. Lemmon, K. Albrecht and D. Howe, Biomass Bioenergy, 2015, 74, 162–171 CrossRef CAS.
- S. Feng, Z. Yuan, M. Leitch and C. C. Xu, Fuel, 2014, 116, 214–220 CrossRef CAS.
- S. Karagoz, T. Bhaskar, A. Muto and Y. Sakata, Fuel, 2005, 84, 875–884 CrossRef.
- T. Sakaki, M. Shibata, T. Sumi and S. Yasuda, Ind. Eng. Chem. Res., 2002, 41, 661–665 CrossRef CAS.
- X. F. Sun, R. Sun, P. Fowler and M. S. Baird, J. Agric. Food Chem., 2005, 53, 860–870 CrossRef CAS PubMed.
- A. Demirbas, Energy Convers. Manage., 2000, 41(15), 1601–1607 CrossRef CAS.
- C. Zhong and X. Wei, Energy, 2004, 29, 1731–1741 CrossRef CAS.
- M. Dietrich, M. Ante and O. Faix, Bioresour. Technol., 1992, 40(2), 171–177 CrossRef.
- Y. H. Chan, et al., J. Supercrit. Fluids, 2014, 95, 407–412 CrossRef CAS.
- P. Das, M. Dinda, N. Gosai and S. Maiti, Energy Fuels, 2015, 29, 4311–4320 CrossRef CAS.
- W. Chen, et al., Appl. Energy, 2014, 128, 209–216 CrossRef CAS.
- S. E. Kinata, K. Loubar, M. Paraschiv, M. Tazerout and C. Belloncle, Appl. Energy, 2014, 115, 57–64 CrossRef CAS.
- Z. Zhu, L. Rosendahl, S. S. Toor, D. Yu and G. Chen, Appl. Energy, 2015, 137, 183–192 CrossRef CAS.
- J. Gan and W. Yuan, Appl. Energy, 2013, 103, 350–357 CrossRef CAS.
- R. Mahadevan, R. Shakya, S. Neupane and S. Adhikari, Energy Fuels, 2015, 29, 841–848 CAS.
- J. J. Meng, A. Moore, D. Tilotta, S. Kelley and S. Park, ACS Sustainable Chem. Eng., 2014, 2, 2011–2018 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1 and S2, Tables S1–S10 can be found in ESI. See DOI: 10.1039/c5ra24760h |
| This journal is © The Royal Society of Chemistry 2016 |