Synthesis and properties of the rapeseed meal-grafted-poly(methyl methacrylate-co-butyl acrylate) oil-absorbents
Abstract
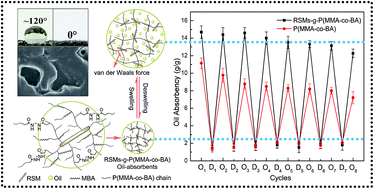
Owing to the unique properties such as oil body (OB) membranes and cellulose components within the structures of rapeseed meals (RSMs), the novel rapeseed meal-grafted-poly(methyl methacrylate-co-butyl acrylate) (RSMs-g-P(MMA-co-BA)) oil-absorbents were prepared and used for oil/water separation in the present study. Specifically, the RSMs-g-P(MMA-co-BA) were successfully synthesized through free radical graft copolymerization from RSMs, methyl methacrylate (MMA) and butyl acrylate (BA) with benzoyl peroxide (BPO) as initiator and N,N′-methylene-bisacrylamide (MBA) as crosslinker. The synthetic mechanism and 3D network structures were confirmed by FTIR and SEM, and the effects of reaction conditions, swelling kinetics, thermodynamics as well as reusability were investigated in detail. The RSMs-g-P(MMA-co-BA) demonstrated good absorption capacity for both organic solvents and oils, and the change of oil absorbency after eight repeated cycles of swelling/deswelling was marginal. Furthermore, the RSMs-g-P(MMA-co-BA) could selectively remove oils from water rapidly, which is promising for their potential applications in oil spill cleanup.

 Please wait while we load your content...
Please wait while we load your content...