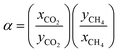Selective separation of CO2–CH4 mixed gases via magnesium aminoethylphosphonate nanoparticles
Sara Simonatoa,
Jens Möllmerb,
Marcus Langeb,
Roger Gläserb,
Reiner Staudt*c and
Claus Feldmann*a
aInstitut für Anorganische Chemie, Karlsruhe Institute of Technology (KIT), Engesserstraße 15, D-76131 Karlsruhe, Germany. E-mail: claus.feldmann@kit.edu; Tel: +49-721-60842855
bInstitut für Nichtklassische Chemie e.V., Permoser Straße 15, 04318 Leipzig, Germany. E-mail: moellmer@inc.uni-leipzig.de
cHochschule Offenburg, Badstraße 24, 77652 Offenburg, Germany. E-mail: reiner.staudt@hs-offenburg.de; Tel: +49-781-205161
First published on 22nd January 2016
Abstract
Magnesium 2-aminoethylphosphonate (Mg2O(2AEP) × 4H2O) nanoparticles (particle diameter: 20–30 nm; specific surface area: 360 m2 g−1) are presented for selective separation of CO2 and CH4. Due to the base amino function, the nanoparticles can reversibly absorb CO2 with a maximal uptake of 153 mg g−1. Absorption and desorption are studied by infrared spectroscopy as well as by gravimetric sorption analysis. Furthermore, Mg2O(2AEP) × 4H2O shows reversible selective separation of CO2 from CH4. Here, pure and mixed gas adsorption isotherms (25 °C, 25 bar) of CO2 and CH4 show maximal uptakes of 153 mg g−1 (CO2) and 15 mg g−1 (CH4). Especially, data of mixed gas isotherms are comparably rare, but highly relevant for material characterization. Experimental isotherms were fitted by a dual-site Langmuir isotherm model (CO2) and a Tòth model (CH4). Mixed adsorption isotherms were modelled by volumetric-chromatographic methods resulting in a selectivity of α = 8 to 20.
Introduction
CO2 is often present in natural gas reserves as a contaminant and needs to be removed as it contributes to the reduction of the energy content of methane and as it increases pipeline corrosion.1 A routinely utilized strategy for separating CO2 from natural gas (the so-called natural gas sweetening process) relates to chemical solvents as sorbents, including the technically most widely applied aqueous diethanolamine.2 This process, however, has two major disadvantages: (i) liquid sorbents are highly corrosive (especially at increased temperature) and require special construction materials; (ii) liquid sorbents require expensive regeneration after each sorption cycle.3 Consequently, solid adsorbents are promising alternatives for CO2 sorption and selective separation of CO2/CH4 mixtures.Three different types of solid adsorbents have been already proposed for CO2/CH4 separation: zeolites,3 activated carbons4 and metal–organic frameworks (MOFs).5 Hereof, zeolites, in particular zeolite 13X and zeolite 5A, show the highest selectivity during equilibrium conditions.3 However, it must be emphasized that the experimental data for these adsorbents are only available for very low CO2 concentrations (gas phase CO2 molar fraction yCO2 = 0.014). Natural gases and biogases, in contrast, often contain more than 40% CO2 and N2.6 Moreover, zeolites have the disadvantage of rapid saturation due to the water vapour being present in natural gas, reducing the CO2 adsorption capacity.7 Finally, they need high temperatures (>300 °C) for regeneration, which increases the total-energy demand for the separation process. Nowadays, the investigation of gas sorption and separation processes is dominated by MOFs as powerful adsorbent materials.6,8 Whereas high selectivity typically cannot be achieved just based on the unprecedented specific surface area, especially, amino-functionalized MOFs exhibit high selectivity for CO2 separation.6,8 Most often, studies relate to pure gases at low pressure (1 bar and below) and low temperature (room temperature and below). Moreover, the presence of humidity is a serious limitation since the availability and adsorption of H2O drastically reduces the CO2 uptake.
Here, we present the inorganic–organic hybrid nanomaterial magnesium aminoethylphosphonate (Mg2O(2AEP) × 4H2O) as a new adsorbent for selective adsorption of CO2 and separation from CH4. The adsorption of CO2 on Mg2O(2AEP) × 4H2O and its reversibility are investigated in detail. Moreover, the CO2/CH4 selectivity is studied based on experimental pure-gas and mixed-gas isotherms. Moreover, gas sorption is modelled based on a dual-site Langmuir isotherm model (CO2) and a Tòth model (CH4). Especially, mixed-gas isotherms are rarely reported in the literature but highly relevant for evaluating the sorption characteristics and selectivity of new materials.
Experimental
Synthesis
For preparing Mg2O(2AEP) × 4H2O nanoparticles, Mg(n-C4H9)2 solution in n-heptane (>99%, Sigma-Aldrich, Germany) and 2-aminoethyl phosphonic acid (H2(2AEP), 99%, Acros Organics, Germany) were used as the starting materials. A transparent microemulsion was prepared by admixing of 70 mL of toluene as the non-polar phase, 3 mL of a solution of 0.27 mM of H2(2AEP) in water as the polar phase, 1.82 g of cetyltrimethylammonium bromide (CTAB, 99%, Sigma) as the surfactant and 5 mL of n-hexane as the co-surfactant. This microemulsion system was stirred vigorously for 30 min at 35 °C (heating via oil bath). Subsequently, a solution of 1.6 mL of 0.5 M Mg(n-C4H9)2 in n-heptane was injected. The formation of nanoparticles started immediately. The resulting suspension was left under stirring at room temperature for additional 12 hours. Thereafter, the nanoparticles were washed three times by sequential centrifugation/redispersion in/from ethanol. Further details regarding synthesis and fundamental materials characterization can be found elsewhere.9Analytical methods
Scanning electron microscopy (SEM) was conducted on a Zeiss Supra 40 VP, using an acceleration voltage of 4 kV and a working distance of 4 mm. SEM samples were prepared by evaporation of an ethanolic suspension of the as-prepared powder. The mean particle diameter was calculated by statistical evaluation of at least 500 particles.X-ray powder diffraction (XRD) was carried out with a Stoe Stadi-P diffractometer using Ge-monochromatized Cu-Kα1 radiation.
Fourier-transformed infrared spectroscopy (FT-IR) was performed on a Bruker Vertex 70. Samples were prepared by pestling of 2 mg of Mg2O(2AEP) × 4H2O with 300 mg of dried KBr in a glove-box.
Inductively coupled plasma atomic emission spectroscopy (ICP-AES) was performed by a commercial analytical laboratory (Microanalytical Laboratory Pascher, Germany) to determine the magnesium and phosphorus content of Mg2O(2AEP) × 4H2O. Moreover, the nitrogen content was determined by combustion analysis.
Thermogravimetry (TG) was performed with a Netzsch STA 409C instrument, applying α-Al2O3 as crucible material as well as reference sample. The samples were heated in air up to 1300 °C with a heating rate of 1 K min−1. The total sample weight was 30 mg.
Elementary analysis (C, H, N) was performed with an Elementar Vario EL device (Elementar, Hanau, Germany).
Gas sorption techniques
Volumetric N2 sorption analysis of as-prepared Mg2O(2AEP) × 4H2O nanoparticles was carried out with a Belsorp mini II. Specific surface and pore volume were determined according to the formalisms given by Brunauer–Emmett–Teller and Barrett–Joyner–Halenda.Gravimetric CO2 and N2 sorption analysis were carried out with a magnetic suspension balance (Rubotherm) that can be operated up to 200 bar. The significance of the balance is ≤0.1 mg. Thus, a stainless steel sample holder was filled with the as-prepared powder sample and the balance was evacuated for 6 h at 333 K and 10−3 mbar until constant mass was achieved. Afterwards, the gas was dosed into the balance chamber to elevated pressures. Equilibrium was achieved within 30 min to 2 h and identified by constant weight and pressure. The temperature was kept constant with an accuracy of ±0.5 K for each measurement. Additionally, a helium buoyancy correction was used to calculate the surface excess mass from the measured values. A detailed description of this procedure can be found elsewhere.4–10
To investigate the uptake of CO2/CH4 gas mixtures, a volumetric-chromatographic method was employed.10–17 A home-made apparatus was used to measure the adsorption equilibria at room temperature and at 1, 5 and 20 bar of pressure under different gas-phase compositions (yCO2 = 0.05, yCO2 = 0.25, yCO2 = 0.75). The apparatus contained different vessels that were filled with the pure gases. To homogenize the gas mixture, a circulation pump was used. A more complete description of the apparatus can be found elsewhere.11–18 The Mg2O(2AEP) × 4H2O nanoparticles were filled into the sample chamber and activated in vacuum at 80 °C for 12 hours. Afterwards, the temperature was fixed at 25 °C, and both pure gases were filled sequentially into the manifolds. In order to ensure homogeneity, a gas-chromatographic analysis was performed prior to starting the adsorption experiment. Subsequently, the valves to the sample cell were opened, and the adsorption took place while circulating the gas phase. After finishing the adsorption step, the circulation was terminated and the sample cell was closed. Gas phase concentrations were then analysed by gas chromatography (Chrompack GC CP 9001; separation column CarboPlot P7; 25 m to 0.53 mm) with sampling from the manifold. With the knowledge of pressure, temperature and gas-phase concentration, the surface-excess amount of both gases, CO2 and CH4, was calculated via the mass balance.
Results and discussion
Synthesis of Mg2O(2AEP) × 4H2O nanoparticles
To obtain Mg2O(2AEP) × 4H2O nanoparticles with high specific surface area as needed for efficient gas sorption and separation, we have performed the particle nucleation in the volume-restricted water pool of a water-in-oil microemulsion.9 Based on this measure, the particle size of Mg2O(2AEP) × 4H2O results in about 20–30 nm at narrow size distribution (Fig. 1). This diameter is in accordance with the data of scanning electron microscopy (SEM) and dynamic light scattering (DLS).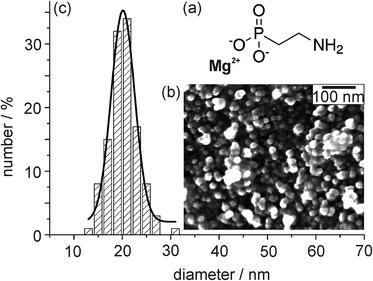 | ||
| Fig. 1 Chemical composition (a) and particle size (according to electron microscopy (b) and dynamic light scattering (c)) of the as-prepared Mg2O(2AEP) × 4H2O nanoparticles.9 | ||
The essential functions of Mg2O(2AEP) × 4H2O comprise the base amino group of the organic 2-aminoethylphosphonate anion that guarantees for effective adsorption of the acidic CO2. The phosphonate group of the [AEP]2− anion, furthermore, allows obtaining an insoluble compound in water, which is prerequisite for preparing nanoparticles. Mg2+ was selected in view of its light weight and its frequently discussed second-order effects regarding the absorption and coordination of CO2. Previous studies have already addressed aminoalkylphosphonates exhibiting different alkyl-chain lengths.9 This included aminomethyl phosphonate [AMP]2−, 1-aminoethyl phosphonate [1AEP]2−, 2-aminoethyl phosphonate [2AEP]2−, aminopropyl phosphonate [APP]2−, and aminobutyl phosphonate [ABP]2−. Hereof, Mg2O(2AEP) × 4H2O turned out as the optimal compound for CO2 sorption.9 To this concern, longer alkyl chains increase the basic character of the amino group, which is preferred for CO2 sorption, in principle. By lengthening the alkyl-chain, on the other hand, the molecular weight of the relevant compound is increased, which reduces the uptake of CO2 in relation to the weight of the sorbent. As a consequence of these contradicting trends, Mg2O(2AEP) × 4H2O results as the most promising nanomaterial for selective CO2–CH4 separation.
According to X-ray powder diffraction Mg2O(2AEP) × 4H2O turned out as totally non-crystalline, which is an advantage and a disadvantage at the same time. Due to the non-crystallinity the porosity and diffusion of small molecules into the nanoparticle core can be expected to be much higher as compared to a periodically ordered, thus crystalline nanomaterial. This is definitely preferred for gas sorption and separation. On the other hand, the chemical characterization of the non-crystalline Mg2O(2AEP) × 4H2O is more difficult since X-ray diffraction is not applicable. To determine the chemical composition of the Mg2O(2AEP) × 4H2O nanoparticles, proving the presence of the functional organic [2AEP]2− anion and of the inorganic [Mg2O]2+ cation is naturally most important. Here, the presence of [2AEP]2− was proven by infrared spectroscopy (FT-IR) (Fig. 2a). The characteristic vibrations of aminoethylphosphonate are clearly visible: ν(O–H): 3300–2900 cm−1; ν(C–H): 2950–2850 cm−1; ν(C–N)/δ(C–H): 1600–1200 cm−1; ν(PO3): 1200–850 cm−1, δ(PO3): 750–400 cm−1. Moreover, the presence of magnesium as well as a Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio of 2.4
P ratio of 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 were validated by inductively coupled plasma atomic emission spectroscopy (ICP-AES). The total thermal weight loss as determined by thermogravimetry (TG) (experimental: 40%; calculated: 41%, Fig. 2b) as well as elemental analysis (EA) (experimental: C: 10 wt%, H: 5.3 wt%, N: 4.1 wt%; calculated: C: 9 wt%, H: 5.4 wt%, N: 5.4 wt%), finally, lead to the composition [Mg2O]2+[2AEP]2− × 4H2O. Details regarding the chemical characterization were reported elsewhere.9
1.0 were validated by inductively coupled plasma atomic emission spectroscopy (ICP-AES). The total thermal weight loss as determined by thermogravimetry (TG) (experimental: 40%; calculated: 41%, Fig. 2b) as well as elemental analysis (EA) (experimental: C: 10 wt%, H: 5.3 wt%, N: 4.1 wt%; calculated: C: 9 wt%, H: 5.4 wt%, N: 5.4 wt%), finally, lead to the composition [Mg2O]2+[2AEP]2− × 4H2O. Details regarding the chemical characterization were reported elsewhere.9
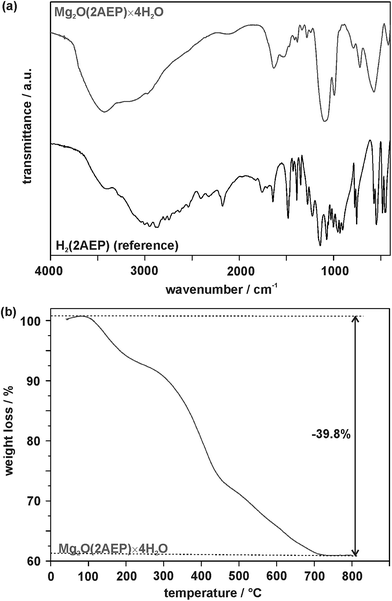 | ||
| Fig. 2 Infrared spectroscopy (a) and thermogravimetry (b) of the as-prepared Mg2O(2AEP) × 4H2O nanoparticles (FT-IR spectra with H2(2AEP) as a reference). | ||
In addition to the particle size and the chemical composition of the Mg2O(2AEP) × 4H2O nanoparticles, the fundamental material characterization addressed the specific surface area and porosity of the sample. To this concern, volumetric nitrogen sorption studies were performed and evaluated according to the Brunauer–Emmett–Teller (BET) and the Barrett–Joyner–Halenda (BJH) formalism. As a result, a specific surface area of 360(10) m2 g−1 and a pore volume of 0.9(1) cm3 g−1 were determined.
CO2 sorption on Mg2O(2AEP) × 4H2O
Mg2O(2AEP) × 4H2O already showed promising CO2 adsorption (up to 150 mg g−1) at high pressure (up to 150 bar) and high temperature (up to 80 °C), whereas the N2 adsorption is negligible (<1 mg g−1) over the complete pressure range (1–150 bar) (Fig. 3).12 Although promising, however, pure-gas data are of limited significance for the much more relevant gas mixtures since interaction and interdiffusion of different molecules in mixed gases can dramatically affect the sorption processes in comparison to pure gases. Studies on mixed gases, on the other hand, are generally limited in the literature, which can be ascribed to the more complex sorption equipment and the more advanced analysis.In general, the CO2 sorption on Mg2O(2AEP) × 4H2O nanoparticles can be understood based on the following equilibrium:
| R–NH2 + CO2 ⇆ [R–NHCOO]− + [R–NH3]+, (R: alkyl group) | (1) |
This reaction of acidic CO2 with the base amino functions of the nanoparticles is well-known from conventional liquid-amine CO2 absorbers (e.g. 2-aminoethanol/MEA, N-methyldiethanolamine/MDEA, 2-amino-2-methyl-1-propanol/AMP) as well as from post-synthesis amine-functionalized MOFs.6,13 Following this reaction, Mg2O(2AEP) × 4H2O shows a maximum uptake of 153 mg g−1 at high pressure (150 bar) (Fig. 3). This value even exceeds the expected amount of 85 mg g−1 that was calculated according to eqn (1). The gravimetric CO2 adsorption and desorption isotherms, moreover, show the principle reversibility of Mg2O(2AEP) × 4H2O. The thermal stability of Mg2O(2AEP) × 4H2O, in addition, is validated by thermogravimetry showing no significant weight loss in the 20–100 °C temperature range (Fig. 2b), which is more relevant for reversible CO2 sorption.
Reversibility and stability of the Mg2O(2AEP) × 4H2O nanoparticles were furthermore tested before and after pure-gas CO2 sorption. In view of the significance (≤0.1 mg) of the magnetic suspension balance, a certain mismatch between the 1st adsorption isotherm (starting at 0 mg g−1) and the 1st desorption isotherm (finished at a residual uptake of about 19 mg g−1) becomes indicative (Fig. 3). Obviously, a certain amount of CO2 remains adsorbed on Mg2O(2AEP) × 4H2O subsequent to the 1st sorption cycle. This view is validated by performing a 2nd sorption cycle instantaneously after the 1st cycle. The 2nd adsorption isotherm starts at 19 mg g−1 and also ends at 19 mg g−1 with the 2nd desorption isotherm (Fig. 3). Hence, the 2nd CO2 sorption cycle is fully reversible. Further sorption cycles show an identical behavior as the 2nd sorption cycle.
Based on infrared spectra, the origin of the residual weight of 19 mg g−1 after the 1st CO2 sorption cycle can be clearly attributed to remaining CO2 (Fig. 4). Hence, the intensity of ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) vibration (1650–1500 cm−1) is significantly increased after the 1st sorption cycle as compared to the as-prepared Mg2O(2AEP) × 4H2O nanoparticles (Fig. 4). It is to be noted that the spectra were normalized on the phosphonate valence vibration with its maximal intensity at 1099 cm−1 since ν(PO3) is independent of the CO2 sorption. Based on this normalization, the intensity of ν(C
O) vibration (1650–1500 cm−1) is significantly increased after the 1st sorption cycle as compared to the as-prepared Mg2O(2AEP) × 4H2O nanoparticles (Fig. 4). It is to be noted that the spectra were normalized on the phosphonate valence vibration with its maximal intensity at 1099 cm−1 since ν(PO3) is independent of the CO2 sorption. Based on this normalization, the intensity of ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) vibration can be directly compared and is now indicative for the CO2 content in Mg2O(2AEP) × 4H2O before and after the CO2 sorption. The finding of CO2 remaining on Mg2O(2AEP) × 4H2O as well as the excess CO2 uptake (85 mg g−1) can be rationalized based on the following reaction (eqn (2)), which is also well-known from conventional liquid-amine CO2 absorbers (e.g. 2-aminoethanol/MEA, N-methyldiethanolamine/MDEA, 2-amino-2-methyl-1-propanol/AMP) as well as from post-synthesis amine-functionalized MOFs.6,13 Molar quantities of water that are intrinsically available in Mg2O(2AEP) × 4H2O support the following reaction and reformation of amine-functionalities.
O) vibration can be directly compared and is now indicative for the CO2 content in Mg2O(2AEP) × 4H2O before and after the CO2 sorption. The finding of CO2 remaining on Mg2O(2AEP) × 4H2O as well as the excess CO2 uptake (85 mg g−1) can be rationalized based on the following reaction (eqn (2)), which is also well-known from conventional liquid-amine CO2 absorbers (e.g. 2-aminoethanol/MEA, N-methyldiethanolamine/MDEA, 2-amino-2-methyl-1-propanol/AMP) as well as from post-synthesis amine-functionalized MOFs.6,13 Molar quantities of water that are intrinsically available in Mg2O(2AEP) × 4H2O support the following reaction and reformation of amine-functionalities.
| [R–NHCOO]− + H2O ⇆ [HCO3]− + R–NH2, (R: alkyl group) | (2) |
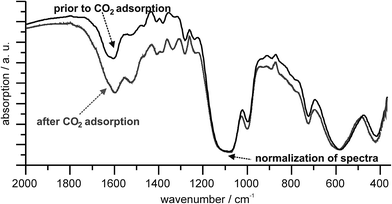 | ||
| Fig. 4 FT-IR spectra of Mg2O(2AEP) × 4H2O nanoparticles before and after CO2 adsorption (spectra normalized on ν(PO3) at 1099 cm−1). | ||
Taking eqn (1) and (2) together, a maximum CO2 uptake of 170 mg g−1 is possible on Mg2O(2AEP) × 4H2O and almost realized with the here observed 153 mg g−1. As a matter of fact, however, total CO2 release from hydrogen carbonate ([HCO3]−, eqn (2)) requires more energy as compared to the carbamate ([R–NHCOO]−, eqn (1)), which also explains the higher energy (i.e., evacuation or heating) that is necessary for complete reversibility.
Complete reversibility of Mg2O(2AEP) × 4H2O and complete release of all absorbed CO2 – including the 19 mg g−1 of CO2 that remain absorbed after the 1st sorption cycle – is nevertheless possible. Considering the fact that the Mg2O(2AEP) × 4H2O nanoparticles are still in contact with a CO2 atmosphere at 1 bar after the 1st sorption cycle, removing the CO2 atmosphere and evacuation might be one option. As the decomposition of [CO3]2− according to eqn (2) requires a higher energy for activation, heating of the Mg2O(2AEP) × 4H2O nanoparticles in nitrogen could be another option.
Indeed evacuation as well as heating allow desorbing largely all CO2 from the Mg2O(2AEP) × 4H2O nanoparticles. To this concern, evacuation was performed at elevated temperature (10−3 mbar, 333 K, 6 h) subsequent to the 1st sorption cycle (Fig. 5a). Alternatively, the CO2 atmosphere can be replaced by helium after the 1st sorption cycle with the Mg2O(2AEP) × 4H2O nanoparticles heated thereafter (1 bar He atmosphere, 353 K, 12 h) (Fig. 5b). Via both measures 13 mg g−1 (evacuation) and 16 mg g−1 (heating in He atmosphere) of CO2 can be removed. Notably the pressure reduction during evacuation needs to be performed slowly (30 min) to avoid dusting inside of the suspension balance. In sum, 96–98% of CO2 (i.e. 146 to 149 mg g−1 of 153 mg g−1) adsorbed on the Mg2O(2AEP) × 4H2O nanoparticles can be reversibly desorbed when including additional evacuation or heating. At least 88% of CO2 (i.e. 134 mg g−1 of 153 mg g−1) can be reversibly adsorbed and desorbed just by pressure swing cycles between 1 and 110 bar.
CO2–CH4 pure gas isotherms
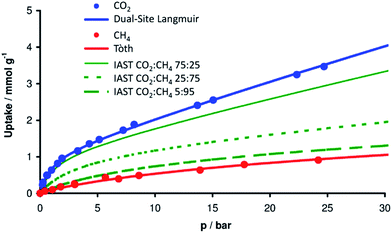
Aiming at CO2 and CH4 sorption and separation, first of all, pure-gas adsorption isotherms of Mg2O(2AEP) × 4H2O were measured gravimetrically at 25 °C (Fig. 6). At 25 bar, the nanoparticles adsorb 3.47 mmol g−1 (153 mg g−1) of CO2, whereas they only absorb 0.9 mmol g−1 (14.5 mg g−1) CH4 under similar conditions. Acidic CO2 is preferably adsorbed on Mg2O(2AEP) × 4H2O nanoparticles over CH4 due to chemisorption on the base amino-groups present in Mg2O(2AEP) × 4H2O (eqn (1)). In contrast to CO2 but similar to N2, CH4 is excluded from this sorption mechanism.Although the adsorption characteristics of pure CO2 and pure CH4 are promising, data of CO2/CH4 gas mixtures are needed for applied process design. To model and predict binary adsorption equilibria from pure gas adsorption isotherms, we have applied the ideal adsorbed solution theory (IAST) first.14 To this regard, a Tòth model15 turned out to be suitable for the description of pure CH4 isotherms, whereas the experimental data for CO2 were fitted using a dual-site-Langmuir model16 (Fig. 6). These two models were used within the IAST, and the calculated data were compared with experimental sorption isotherms from CO2/CH4 mixed gas systems. In Fig. 6, the green lines represent the IAST calculation of three different CO2/CH4 mixtures with yCO2 = 0.05, yCO2 = 0.25 and yCO2 = 0.75.
CO2–CH4 mixed gas isotherms
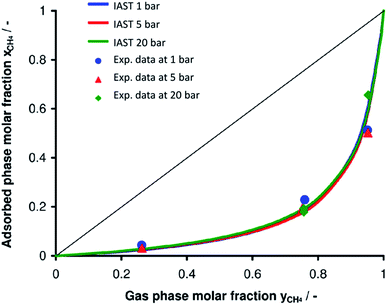
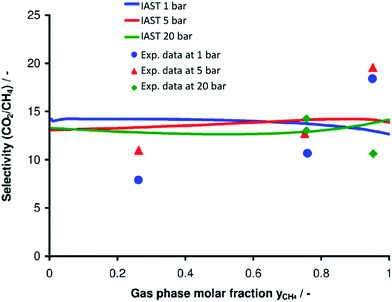
Next, the adsorption of CO2 and CH4 mixtures was experimentally investigated at room temperature and at three different constant pressures (1, 5, 20 bar) by volumetric-chromatographic measurements. Here, the prediction from IAST and the experimental data are in excellent agreement (Fig. 7). Over the whole range of pressures and gas mixtures, adsorption of CO2 on Mg2O(2AEP) × 4H2O is preferred over CH4, confirming the interaction of CO2 with the amino-groups available in the nanomaterial.6 To evaluate the potential of Mg2O(2AEP) × 4H2O regarding CO2/CH4 separation, the selectivity (α) was considered which is defined by:herein, x is the molar fraction of the respective gas in the adsorbate; y is the molar fraction of the respective gas in the gas phase. The dimensionless selectivity indicates how much CO2 is enriched in the adsorbate (xCO2) as a function of the gas-phase composition at constant pressure and temperature. If α is higher than 1, CO2 is preferentially adsorbed. On the contrary, CH4 adsorption is preferred if α is lower than 1. For Mg2O(2AEP) × 4H2O nanoparticles, the experimental as well as the theoretical adsorption show nearly constant selectivity at low and high CO2 gas phase molar fractions, ranging from α = 8 to α = 20 (Fig. 8). The IAST predictions are well in agreement with the experimental data and show values of α between 11 and 15.
When evaluating the sorption properties of the Mg2O(2AEP) × 4H2O nanoparticles, the CO2 selectivity for CO2/CH4 mixtures at high CO2 content (>20%) exceeds many known materials, including zeolites, activated carbons and MOFs (Table 1). With regard to MOFs, only the amino-functionalized MOFs MIL-101(Al) and NH2-MIL-53(Al), especially at low pressure (<10 bar) show a selectivity higher than Mg2O(2AEP) × 4H2O.5e,f On the other hand, MOFs often need advanced synthesis. Moreover, they are sensitive to humidity (i.e. blocking the CO2 sorption) and partly hydrolyzed by water (i.e. restricting the chemical stability).17 On the contrary, Mg2O(2AEP) × 4H2O nanoparticles are prepared in water. In contrast to zeolites and activated carbons, moreover, Mg2O(2AEP) × 4H2O can be easily regenerated since the absorbed CO2 is desorbed either by slight heating (80 °C) and or by decompression.
| Adsorbent | Adsorption conditions | Gas mixture composition | Selectivity α (CO2/CH4) | Pure CO2 uptake | Ref. |
|---|---|---|---|---|---|
| a † = excess adsorption; ‡ = absolute adsorption; †‡ = reference is not clear in whether absolute or excess adsorption is given. | |||||
| Mg2O(2AEP) × 4H2O | 1–20 bar, 25 °C | yCO2 = 0.05 | 8–20 | 153 mg g−1, (25 °C, 25 bar)† | This study |
| yCO2 = 0.25 | |||||
| yCO2 = 0.75 | |||||
| HKUST-1, (Cu3(BTC)2) | 1–10 bar, 30 °C | yCO2 = 0.25 | 5–12 | 616 mg g−1, (30 °C, 40 bar)‡ | 5a |
| yCO2 = 0.50 | |||||
| yCO2 = 0.75 | |||||
| Zn2(ndc)2(dpni) | 0.1–10 bar, 23 °C | yCO2 = 0.5 | 4–30 | 220 mg g−1, (23 °C, 18 bar)† | 5b |
| MIL-53(Cr) | 10 bar, 30 °C | yCO2 = 0.25, 0.50, 0.75 | 2–15 | 500 mg g−1, (30 °C, 2 bar)†‡ | 5c |
| MIL-100(Cr) | 1–10 bar, 30 °C | yCO2 = 0.25, 0.50, 0.75 | 6–8 | 350 mg g−1, (30 °C, 10 bar)†‡ | 5d |
| NH2-MIL-53(Al), (amino-funct.) | 1/5/45 bar, 30 °C | yCO2 = 0.4 | 60–100/20/2–3 | 310 mg g−1, (30 °C, 40 bar)†‡ | 5e |
| MIL-101(Al), (amino-funct.) | 1 bar, 25 °C | yCO2 = 0.2 | 15–60 | 620 mg g−1, (25 °C, 30 bar)†‡ | 5f |
| TB-MOF, ([Me2NH2][Tb3L3(HCOO) × DMF × 15H2O]) | 0.1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bar, 20 °C bar, 20 °C |
yCO2 = 0.5 | 11–17 | 78 mg g−1, (20 °C, 1 bar) | 18a |
| [Cu3(CPT)4(μ3-OH)] × NO3 × 7H2O | 0.1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bar, 25 °C bar, 25 °C |
yCO2 = 0.5 | 18–36 | 118 mg g−1, (25 °C, 1 bar) | 18b |
| Zeolite 13X, (NaX) | 10–40 bar, 25 °C | yCO2 = 0.014 | 140–160 | 145 mg g−1, (50 °C, 1 bar) | 3 and 6 |
| 325 mg g−1, (25 °C, 32 bar)†‡ | |||||
| Zeolite 5A, (CaA) | 10–40 bar, 25 °C | yCO2 = 0.014 | 250–300 | 150 mg g−1, (30 °C, 1 bar) | 3 and 19 |
| Active carbon, (AC A35/4) | 1–15 bar, 25 °C | yCO2 = 0.2 | 3–9 | 352 mg g−1, (20 °C, 10 bar) | 4 |
| yCO2 = 0.5 | |||||
| yCO2 = 0.9 | |||||
| Boron nitride | >227 °C | CO2, CH4, H2 | >10 | 80 kcal mol−1 adsorption energy | 20 |
Conclusions
In conclusion, the inorganic–organic hybrid nanomaterial Mg2O(2AEP) × 4H2O is a highly promising alternative to existing adsorbents for CO2 and selective separation from CH4. The experimental data of different CO2/CH4 gas mixtures and theoretical adsorption models show nearly constant selectivity at low as well as at high CO2 gas phase molar fractions ranging from α = 8 to 20. For the first time, adsorption and selectivity of CO2/CH4 mixtures (yCO2 = 0.05, 0.25, 0.75) are experimentally determined and modelled based on a dual-site Langmuir isotherm model (CO2) and a Tòth model (CH4). The results from both approaches are in good agreement. Based on these results, Mg2O(2AEP) × 4H2O nanoparticles may gain considerable interest for selective separation of CO2 as a contaminant in natural gas. The solid nanomaterial possesses basic properties and can be used in alternative to the widely applied but corrosive liquid amines. Mg2O(2AEP) × 4H2O is even more interesting due to its uncomplex synthesis in water, its low sensitivity to water, and its straightforward regeneration via gentle heating and/or decompression.Acknowledgements
The authors are grateful to the Deutsche Forschungsgemeinschaft (DFG) for funding of analytical equipment.Notes and references
- Y. S. Bae, O. K. Farha, A. M. Spokoyny, C. A. Mirkin, J. T. Hupp and R. Q. Snurr, Chem. Commun., 2008, 35, 4135 RSC.
- G. Astarita, D. W. Savage and A. Bisio, Gas treating with chemical solvents, Wiley, Chichester, 1983 Search PubMed.
- P. D. Rolniak and R. Kobayashi, AIChE J., 1980, 26, 616 CrossRef CAS.
- M. Heuchel, G. M. Davies, E. Buss and N. A. Seaton, Langmuir, 1999, 15, 8695 CrossRef CAS.
- (a) L. Hamon, E. Jolimaitre and G. D. Pirngruber, Ind. Eng. Chem. Res., 2010, 49, 7497 CrossRef CAS; (b) Y. Bae, K. Mulfort, H. Frost, P. Ryan, S. Punnathanam, L. Broadbelt, J. T. Hupp and R. Q. Snurr, Langmuir, 2008, 24, 8592 CrossRef CAS PubMed; (c) L. Hamon, P. L. Llewellyn, T. Devic, A. Ghoufi, G. Clet, V. Guillerm, G. D. Pirngruber, G. Maurin, C. Serre, G. Driver, W. van Beek, E. Jolimaıtre, A. Vimont, M. Daturi and G. Ferey, J. Am. Chem. Soc., 2009, 131, 17490 CrossRef CAS PubMed; (d) L. Hamon, N. Heymans, P. L. Llewellyn, V. Guillerm, A. Ghoufi, S. Vaesen, G. Maurin, C. Serre, G. De Weireld and G. D. Pirngruber, Dalton Trans., 2012, 41, 4052 RSC; (e) S. A. Peter, G. V. Baron, J. Gascon, F. Kapteijn and J. F. M. Denayer, Adsorption, 2013, 19, 1235 CrossRef CAS; (f) P. Serra-Crespo, E. V. Ramos-Fernandez, J. Gascon and F. Kapteijn, Chem. Mater., 2011, 23, 2565 CrossRef CAS.
- D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058 CrossRef PubMed.
- G. Li, P. Xiao, P. Webley, J. Zhang and R. Singh, Energy Procedia, 2009, 1, 1123 CrossRef CAS.
- (a) S. Li and F. Huo, Nanoscale, 2015, 7, 7482 RSC; (b) Z. Zhang, Z.-Z. Yao, S. Xiang and B. Chen, Energy Environ. Sci., 2014, 7, 2868 RSC; (c) S. Chaemchuen, N. A. Kabir, K. Zhou and F. Verpoort, Chem. Soc. Rev., 2013, 42, 9304 RSC; (d) X. Wang, H. Li and X.-J. Hou, J. Phys. Chem. C, 2012, 116, 19814 CrossRef CAS.
- J. G. Heck, J. Napp, S. Simonato, J. Möllmer, M. Lange, H. R. Reichardt, R. Staudt, F. Alves and C. Feldmann, J. Am. Chem. Soc., 2015, 137, 7329 CrossRef CAS PubMed.
- (a) J. U. Keller and R. Staudt, Gas Adsorption Equilibria, Springer, Berlin, 2005 Search PubMed; (b) F. Dreisbach and H. W. Losch, J. Therm. Anal. Calorim., 2000, 62, 515 CrossRef CAS; (c) J. Moellmer, A. Moeller, F. Dreisbach, R. Glaeser and R. Staudt, Microporous Mesoporous Mater., 2011, 138, 140 CrossRef CAS.
- J. Möllmer, M. Lange, A. Möller, C. Patzschke, K. Stein, D. Lässig, J. Lincke, R. Gläser, H. Krautscheid and R. Staudt, J. Mater. Chem., 2012, 22, 10274 RSC.
- P. Leidinger, S. Simonato and C. Feldmann, Chem. Commun., 2012, 48, 7046 RSC.
- (a) S. Choi, J. H. Drese and C. W. Jones, ChemSusChem, 2009, 2, 796 CrossRef CAS PubMed; (b) S. Ma and H. C. Zhou, Chem. Commun., 2010, 46, 44 RSC; (c) D.-Y. Hong, Y. K. Hwang, C. Serre, G. Ferey and J.-S. Chang, Adv. Funct. Mater., 2009, 19, 1537 CrossRef CAS.
- A. L. Myers and J. M. Prausnitz, AIChE J., 1965, 11, 121 CrossRef CAS.
- J. Tòth, Adv. Colloid Interface Sci., 1995, 55, 1 CrossRef.
- P. M. Mathias, R. Kumar, J. D. Moyer, J. M. Schork, S. R. Srinivas-an, S. R. Auvil and O. Talu, Ind. Eng. Chem. Res., 1996, 35, 2477 CrossRef CAS.
- J. Liu, P. K. Thallapally, B. P. McGrail, D. R. Brown and J. Liu, Chem. Soc. Rev., 2012, 41, 2308 RSC.
- (a) Y. Yang, F. Jiang, L. Chen, J. Pang, M. Wu, Y. Wan, J. Pan, J. Qian and M. Hong, J. Mater. Chem. A, 2015, 3, 13526 RSC; (b) D. M. Chen, X. P. Zhang, W. Shi and P. Cheng, Inorg. Chem., 2015, 54, 5512 CrossRef CAS.
- Z. Liu, C. A. Grande, P. Li, J. G. Yu and A. E. Rodrigues, Sep. Sci. Technol., 2011, 46, 434 CrossRef CAS.
- Q. Sun, Z. Li, D. J. Searles, Y. Chen, G. Q. Lu and A. J. Du, J. Am. Chem. Soc., 2013, 135, 8246 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |