{[MIM-NO2]C(NO2)3} a unique nano ionic liquid: application to the synthesis of novel Biginelli-type compounds†
Ardeshir Khazaei*a,
Mohammad Ali Zolfigol*a,
Saied Alaiea,
Saeed Bagherya,
Babak Kaboudinb,
Yadollah Bayatc and
Asiye Asgaric
aDepartment of Organic Chemistry, Faculty of Chemistry, Bu-Ali Sina University, Hamedan 6517838683, Iran. E-mail: zolfi@basu.ac.ir; mzolfigol@yahoo.com; Khazaei_1326@yahoo.com; Fax: +988138257407
bDepartment of Chemistry, Institute for Advanced Studies in Basic Sciences (IASBS), Gava Zang, Zanjan 45137-66731, Iran
cFaculty of Chemistry and Chemical Engineering, Malek Ashtar University of Technology, Tehran, Iran
First published on 18th January 2016
Abstract
1-Methyl-3-nitro-1H-imidazol-3-ium trinitromethanide {[MIM-NO2]C(NO2)3} as a novel and green nano structure ionic liquid (NIL) efficiently catalyzed the synthesis of 3,4-dihydropyrimidin-2(1H)-one derivatives via the one-pot Biginelli-type three-component condensation reaction of aromatic aldehydes, urea and 1,3-dione at room temperature under solvent-free conditions. Some advantages of the presented method are excellent cost effectiveness, effective catalysis and reusability of catalyst. {[MIM-NO2]C(NO2)3} was fully characterized by IR, 1H NMR, 13C NMR, MS, X-ray diffraction patterns (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM), thermal gravimetric (TG) and derivative thermal gravimetric (DTG) analysis. We think that the described novel NIL and other homologs will be suitable sources for energetic materials. Thus the present work can open up a new and promising insight into the rational design, synthesis and applications of nano task-specific ionic liquids (NTSILs) for various green purposes. In the presented work, more than twenty products have been reported for the first time.
Introduction
Multicomponent reactions (MCRs) have developed as effective and influential approaches in modern synthetic organic chemistry due to the synthesis of complex organic molecules from simple and readily beneficial substrates which can be attained in a very fast and effective manner without the isolation of any intermediates.1–3 Subsequently, emerging new MCRs and improving known MCRs are general areas of study in current organic chemistry. One such reaction is the Biginelli-type three-component reaction in the synthesis of 3,4-dihydropyrimidin-2(1H)-one derivatives.The Biginelli-type reaction for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones (DHPMs) which have various pharmacological activities such as antibacterial, antihypertensive activity and antiviral, as well as being efficient α1a-antagonists.4–6 Numerous DHP calcium antagonists have been reported as potential drugs for the therapy of congestive heart failure.7 DHP drugs, specifically amlodipine, nicardipine and nifedipine, are cardiovascular factors for the therapy of hypertension.8 The first synthesis of 3,4-dihydropyrimidin-2(1H)-ones described by Biginelli in 1893 involved a one-pot three-component condensation of a β-ketoester, an aldehyde and urea.9,10 Lately, DHPs have been applied as organocatalysts for asymmetric reactions such as the hydrogenation of α,β-unsaturated aldehydes and ketones,11 asymmetric reductive amination of aldehydes12 and hydrogenation of quinolines in the synthesis of alkaloids.13 Furthermore, dihydropyridines have found uses in stereo specific hydrogen transfer reduction of phenylglyoxylic and pyruvic acid to biomimetic models of lactase dehydrogenase.14 Numerous other approaches have been introduced including the application of tungstophosphoric acid,15 polyvinylsulfonic acid,16 CuI,17 trichloroisocyanuric acid,18 chloroacetic acid19 and H3PMo12O40 (Scheme 1).20
In the past few years, ionic liquids (ILs) have been one of the most studied subjects in academic and industrial fields21,22 important because of their attractive “green” characteristics, for example nonflammablility, high thermal and chemical stabilities, negligible vapor pressure and good reusability.23–26 Furthermore, the designable characteristic offers an alternative significant agent that is responsible for the unique prosperity of ionic liquids.27,28 Their properties can be reformed by changing anions, cations, or alkyl substituents on the cations. Research of greener replacements to conventional organic solvents forms a main part of today’s drive to supportable chemistry.29 Because of their useful and highly modifiable properties, ionic liquids belong amongst the most promising applicants. The advantages of ionic liquids as exchange media in organic synthesis have been confirmed in most examples.30 Many catalysts and reagents have been supported in the ionic liquid phase, resulting in improved reactivities and selectivities in several important transformations;31 due to its versatility, specific consideration in immobilization and derivatization has been dedicated to proline.32 Among these useful solvents, acidic or protic ionic liquids have a wide range of uses.33 Ionic liquids combining a chiral cation or anion can make attractive points of stereoselectivity in a wide range of asymmetric reactions.34
In continuation of our studies on the dinitrogen tetraoxide (N2O4),35 metal nitrate dinitrogen tetroxide complexes,36 [NO+·Crown·H(NO3)2−]37 and also knowledge-based development of task specific ionic liquids (TSILs),38 we found that desirable structural diversity of ILs with especial properties could be achieved via the design and synthesis of novel cationic cores with suitable anionic counterparts. With this aim, we decided to unite all of these research areas to design, synthesis and applications of TSILs. In this regard, we have prepared 1-methyl-3-nitro-1H-imidazol-3-ium trinitromethanide {[MIM-NO2]C(NO2)3} (Scheme 2) and used it in a green, eco-friendly and efficient method for the one-pot Biginelli-type reaction in the three-component synthesis of 3,4-dihydropyrimidin-2(1H)-one (DHPM) derivatives from the reaction of aromatic aldehydes, urea and 1,3-dione under solvent-free conditions at room temperature (Scheme 3).
 | ||
| Scheme 2 The synthesis of 1-methyl-3-nitro-1H-imidazol-3-ium trinitromethanide {[MIM-NO2]C(NO2)3} as a nano ionic liquid (NIL) catalyst. | ||
 | ||
| Scheme 3 The Biginelli-type reaction in the synthesis of 3,4-dihydropyrimidin-2(1H)-one derivatives in the presence of {[MIM-NO2]C(NO2)3} as a novel nano ionic liquid (NIL) catalyst. | ||
Results and discussion
Characterization of 1-methyl-3-nitro-1H-imidazol-3-ium trinitromethanide {[MIM-NO2]C(NO2)3} as a nano ionic liquid (NIL) catalyst
The structure of 1-methyl-3-nitro-1H-imidazol-3-ium trinitromethanide {[MIM-NO2]C(NO2)3} as a nano ionic liquid (NIL) catalyst was studied and identified via FT-IR, 1H NMR, 13C NMR, Mass, TG, DTG, XRD, SEM and TEM analysis.The IR spectrum of the nano ionic liquid catalyst displayed two peaks detected at 1550 cm−1 and 1384 cm−1 linked to vibrational modes of –NO2 bonds. Moreover, the peak detected at 3144 cm−1 related to a C–H stretching group on the imidazolium ring (Fig. 1).
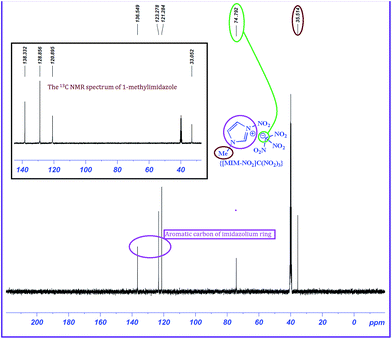
Moreover, the 1H NMR and 13C NMR spectra of the {[MIM-NO2]C(NO2)3} as a NIL in DMSO-d6 are shown in Fig. 2 and 3. The 1H NMR chemical shift changes of {[MIM-NO2]C(NO2)3} in comparison with 1-methylimidazole showed formation of nano ionic liquid catalyst (Fig. 2).
The 13C NMR spectra of {[MIM-NO2]C(NO2)3} as a NIL was another evidence to confirm the structure of it. The peak at 74.8 was linked to the carbon of the –C(NO2)3 group in trinitromethanide. Also, the 13C NMR chemical shift changes of {[MIM-NO2]C(NO2)3} as a NIL rather than 1-methylimidazole depicted formation of the nano ionic liquid catalyst (Fig. 3).
The thermal gravimetric (TG) and derivative thermal gravimetric (DTG) analysis displayed that {[MIM-NO2]C(NO2)3} as a NIL catalyst was very stable and there was no evident mass loss before 205 °C. The important weight loss (74%) between 142 and 222 °C is attributed mainly to the thermal decomposition of the C(NO2)3 counterion moiety. Also the weight loss (26%) occurred in the range of 222–365 °C, being attributed to the loss of the main {[MIM-NO2]C(NO2)3}. The thermal gravimetric analysis of the NIL catalyst presented significant loss in two steps, and decomposed after 365 °C (Fig. 4).
 | ||
| Fig. 4 The thermal gravimetric (TG) and derivative thermal gravimetric (DTG) analysis of {[MIM-NO2]C(NO2)3} as a NIL catalyst. | ||
{[MIM-NO2]C(NO2)3} as a NIL catalyst was studied via X-ray diffractometry (XRD) pattern (Fig. 5), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) (Fig. 6). In an effort to prove that the {[MIM-NO2]C(NO2)3} was appropriately synthesized, firstly, its XRD pattern was recorded. As shown in Fig. 5, the XRD patterns of {[MIM-NO2]C(NO2)3} as a NIL catalyst reveal peaks at 2θ ≈ 19.70°, 26.60°, 27.00°, 28.50°, 29.80° and 40.10°, correspondingly, which was confirmed by the described value of scanning electron microscopy (SEM) and transmission electron microscopy (TEM) (Fig. 6). Peak width (FWHM), size and inter planer distance linked to the XRD pattern of {[MIM-NO2]C(NO2)3} were studied in the 19.70° to 40.10° range and the attained results have been summarized in Table 1. The average crystallite size D was studied using the Debye–Scherrer formula: D = Kλ/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ), K is the Scherrer constant, λ being the X-ray wavelength, β is the half-maximum peak width, and θ is the Bragg diffraction angle. The average size of the NIL catalyst, subsequently, achieved from this equation was found to be about 10.61–62.03 nm, which is fundamentally in accordance with the scanning electron microscopy and transmission electron microscopy (Fig. 6).
θ), K is the Scherrer constant, λ being the X-ray wavelength, β is the half-maximum peak width, and θ is the Bragg diffraction angle. The average size of the NIL catalyst, subsequently, achieved from this equation was found to be about 10.61–62.03 nm, which is fundamentally in accordance with the scanning electron microscopy and transmission electron microscopy (Fig. 6).
 | ||
| Fig. 6 Scanning electron microscopy (SEM) (a) and transmission electron microscopy (TEM) (b, c and d) of {[MIM-NO2]C(NO2)3} as a NIL catalyst. | ||
| Entry | 2θ | Peak width [FWHM] (degree) | Size [nm] | Inter planer distance [nm] |
|---|---|---|---|---|
| 1 | 19.70 | 0.13 | 62.03 | 0.464725 |
| 2 | 26.60 | 0.19 | 42.98 | 0.334710 |
| 3 | 27.00 | 0.77 | 10.61 | 0.329842 |
| 4 | 28.50 | 0.25 | 32.79 | 0.312814 |
| 5 | 29.80 | 0.18 | 45.68 | 0.299456 |
| 6 | 40.10 | 0.28 | 30.21 | 0.224595 |
The mass spectrum of the {[MIM-NO2]C(NO2)3} as a NIL catalyst is in agreement with the structure of the NIL catalyst and displayed the parent peak at 278 m/z (Fig. 7).
Application of 1-methyl-3-nitro-1H-imidazol-3-ium trinitromethanide {[MIM-NO2]C(NO2)3} as a nano ionic liquid (NIL) catalyst
After the synthesis of 1-methyl-3-nitro-1H-imidazol-3-ium trinitromethanide {[MIM-NO2]C(NO2)3} as a nano ionic liquid (NIL) catalyst, to optimize the reaction conditions, we have confirmed the efficiency of the catalyst for the one-pot Biginelli-type reaction in the three-component synthesis of 3,4-dihydropyrimidin-2(1H)-one (DHPM) derivatives (Scheme 3). For this aim, as a model, the condensation reaction of ethyl acetoacetate, urea and naphthalene-1-carbaldehyde was studied in the presence of different amounts of the catalyst at a range of 25–100 °C under solvent-free conditions (Table 2). As Table 2, displays that, the best results were attained when the reaction was achieved using 1 mol% of {[MIM-NO2]C(NO2)3} as a NIL catalyst at room temperature (Table 2, entry 5). In the absence of NIL catalyst, no yield of the product was obtained after 1 h at room temperature and 100 °C (Table 2, entries 1 and 2). Increasing the reaction temperature and catalyst loading did not improve the rate of the reaction (Table 2, entries 6–12).| Entry | Catalyst amount (mol%) | Temperature (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: ethyl acetoacetate (1 mmol), urea (1 mmol), naphthalene-1-carbaldehyde (1 mmol).b Isolated yield. | ||||
| 1 | — | r.t. | 60 | — |
| 2 | — | 100 | 60 | — |
| 3 | 0.5 | r.t. | 30 | 57 |
| 4 | 0.5 | 100 | 30 | 57 |
| 5 | 1 | r.t. | 5 | 97 |
| 6 | 1 | 50 | 5 | 97 |
| 7 | 1 | 75 | 5 | 97 |
| 8 | 1 | 100 | 5 | 97 |
| 9 | 2 | r.t. | 5 | 97 |
| 10 | 2 | 100 | 5 | 97 |
| 11 | 5 | r.t. | 5 | 97 |
| 12 | 5 | 100 | 5 | 97 |
To compare the capability of the solution versus solvent-free conditions, a mixture of ethyl acetoacetate, urea and naphthalene-1-carbaldehyde as a model reaction, using 1 mol% of {[MIM-NO2]C(NO2)3} as a NIL catalyst in different solvents such as H2O, C2H5OH, CH3CN, CH2Cl2, CH3CO2Et, toluene and benzene was studied at room temperature. The results are summarized in Table 3. As shown in Table 3, solvent-free was the best condition in this reaction.
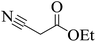
Stimulated by the significant results and with the aim of verification to overview the scope of this original method, several 3,4-dihydropyrimidin-2(1H)-one (DHPM) derivatives were synthesized from a one-pot Biginelli-type three-component condensation reaction of urea, aromatic aldehydes and 1,3-diones under solvent-free conditions at room temperature in the presence of a catalytic amount of {[MIM-NO2]C(NO2)3} as a novel NIL catalyst. The results have been displayed in Table 4. The effect of substituents on the aromatic ring indicates the estimated strong effects in terms of yields under these reaction conditions. All aromatic aldehydes containing electron-releasing substituents and electron-withdrawing substituents on their aromatic ring gave the related products in high to excellent yields in short reaction times and high yields. The reaction times of aromatic aldehydes having electron withdrawing groups were rather faster than the electron donating groups. Furthermore, according to reaction time and isolated yields, the order of reactivity in the 1,3-diones is as follows: ethyl acetoacetate > methyl acetoacetate > dimedone > 1,3-cyclohexanedione > acetyl acetone > ethyl cyanoacetate.
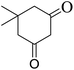
| Entry | Aldehyde | 1,3-Dione | Time (min) | Yieldb (%) | M.p (°C) [Lit.]Ref |
|---|---|---|---|---|---|
| a Reaction conditions: 1,3-dione (1 mmol), urea (1 mmol), aromatic aldehyde (1 mmol).b Isolated yield. | |||||
| 1 |  |
 |
15 | 93 | 240–242 |
| 2 | 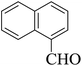 |
 |
15 | 94 | 215–217 |
| 3 | 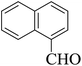 |
 |
10 | 96 | 228–230 |
| 4 | 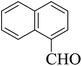 |
 |
5 | 97 | 225–227 |
| 5 |  |
 |
10 | 95 | 219–221 |
| 6 |  |
 |
15 | 95 | 233–235 |
| 7 | 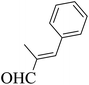 |
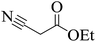 |
30 | 87 | 220–222 |
| 8 |  |
 |
30 | 88 | 209–211 |
| 9 | 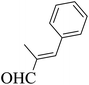 |
 |
25 | 90 | 208–210 |
| 10 |  |
 |
20 | 91 | 192–194 |
| 11 |  |
 |
25 | 89 | 228–230 |
| 12 |  |
 |
30 | 89 | 205–207 |
| 13 |  |
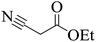 |
20 | 92 | 217–219 |
| 14 |  |
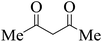 |
20 | 93 | 208–210 |
| 15 |  |
 |
15 | 95 | 235–237 |
| 16 |  |
 |
10 | 96 | 263–265 [289–291]16 |
| 17 | 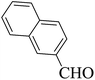 |
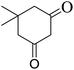 |
15 | 94 | 202–204 |
| 18 | 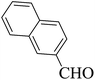 |
 |
20 | 94 | 219–221 |
| 19 |  |
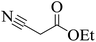 |
25 | 89 | 208–210 |
| 20 | 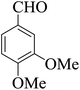 |
 |
25 | 90 | 204–206 |
| 21 |  |
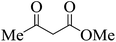 |
20 | 92 | 212–214 |
| 22 |  |
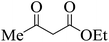 |
15 | 93 | 175–177 [176–178]16 |
| 23 |  |
 |
20 | 91 | 201–203 |
| 24 |  |
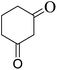 |
25 | 91 | 196–198 |
| 25 |  |
 |
5 | 98 | 208–210 [208–209]16 |
The proposed catalytic approach is displayed in Scheme 4, which is in agreement with literature reports.39 Also Brenno A. D. Neto et al., investigated the Biginelli-type reaction mechanism via spectral studies.40 The formation of DHPM derivatives 6–8 include an acylimine intermediate (11) via the reaction between aldehyde (1) and urea (2) (the rate-determining step), the addition of 1,3-dione (3–5) to the iminium ion (11), and consequent cyclization and dehydration.
Additionally, reusability of the {[MIM-NO2]C(NO2)3} as a NIL catalyst was confirmed on condensation of ethyl acetoacetate, urea and naphthalene-1-carbaldehyde. At the end of the reaction, ethyl acetate was added to the reaction mixture and heated to extract the product and any remaining starting materials. This solution was washed with water to separate the catalyst from other materials (the product is soluble in hot ethyl acetate and the NIL catalyst is soluble in water). The aqueous layer was decanted, separated and applied to an alternative reaction after the removal of water. It was identified that the catalytic activity of the catalyst was restored within the limits of experimental error for four continuous runs (Fig. 8). The recycled catalyst was also characterized by FTIR, 1H NMR and 13C NMR spectra after its application in the reaction. These spectra were identical as those of the fresh catalyst. The deactivation of the nano molten salt catalyst is low. The reaction was scaled up to 10 mmol of ethyl acetoacetate, urea and naphthalene-1-carbaldehyde in the presence of 10 mol% of NMS catalyst at room temperature. The yield of the reaction was 97% after 5 min and 90% after the fourth run. The results are summarized in Fig. 8. Also, the structure of reused {[MIM-NO2]C(NO2)3} was confirmed by IR, 1H NMR and 13C NMR spectra after its application in the reaction. These presented spectra are in agreement with the spectra of fresh catalyst (Fig. S184 to S186†).
Conclusion
In summary, a novel, green and environmentally friendly nano ionic liquid catalyst namely 1-methyl-3-nitro-1H-imidazol-3-ium trinitromethanide {[MIM-NO2]C(NO2)3} was designed, studied and fully characterized by IR, 1H NMR, 13C NMR, MS, thermal gravimetric analysis (TGA), differential thermal gravimetric (DTG), X-ray diffraction patterns (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) analysis. Catalytic application of {[MIM-NO2]C(NO2)3} was studied in the one-pot Biginelli-type reaction in the synthesis of 3,4-dihydropyrimidin-2(1H)-one (DHPM) derivatives via a three-component condensation reaction between urea, aromatic aldehydes and 1,3-diones under solvent-free conditions at room temperature. Various important advantages of this study are reasonably high yield, short reaction time, cleaner reaction profile, reusability of the NIL catalyst and simple work-up. Additionally, the reaction conditions are in close agreement with the green chemistry disciplines. We think that the described novel NIL and other homologs will be suitable sources for energetic materials. Thus the present work can open up a new and promising insight into the rational design, synthesis and applications of nano task-specific ionic liquids (NTSILs) for various green purposes. Since this development of green protocols with cheap materials and simple synthetic procedures is much in demand for sustainable development, further research for systematic and knowledge-based development of this field is ongoing in our research group.Experimental
The materials were purchased from Merck, Fluka and Sigma-Aldrich and were used without any additional purification. All reactions were monitored by thin layer chromatography (TLC) on gel F254 plates. NMR (1H NMR 400 MHz and 13C NMR 100) spectra were recorded in pure deuterated DMSO with tetramethylsilane (TMS) as the internal standard. The synthesized catalyst was characterized by IR, 1H NMR, 13C NMR, MS, X-ray diffraction patterns (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM), thermogravimetric (TG) and derivative thermal gravimetric (DTG) analysis. X-ray diffraction (XRD) patterns of all catalysts were performed on a APD 2000, Ital structure with Cu Kα radiation (λ = 0.1542 nm) operating at 50 kV and 20 mA in a 2 h range of 10–70° with a step size of 0.01° and a time step of 1.0 s to assess the crystallinity of the catalyst. Fourier transform-infrared spectra of the samples were recorded on a Perkin-Elmer FT-IR spectrometer 17259 using KBr disks. Thermogravimetric analyses using a Perkin-Elmer TGA were performed on catalysts. The SEM analyses were performed with a TESCAN/MIRA with a maximum acceleration voltage of the primary electrons between 10 and 15 kV. Transmission electron microscopy (TEM) measurements were carried out on a Philips CM10 analyzer operating at 120 kV.General procedure for the preparation of novel nano ionic liquid catalyst: 1-methyl-3-nitro-1H-imidazol-3-ium trinitromethanide {[MIM-NO2]C(NO2)3}
To a round-bottomed flask (50 mL) containing 1-methylimidazole (3 mmol; 0.246 g) in CH3CN (5 mL), was added tetranitromethane (3 mmol; 0.588 g) dropwise and heated over a period of 30 min at room temperature. Consequently the solvent was removed via distillation under reduced pressure, the product was dried under vacuum at 80 °C for 2 h. An orange solid was produced in quantitative yield (Scheme 2).General procedure for the synthesis of 3,4-dihydropyrimidin-2(1H)-one (DHPM) derivatives
To amixture of aromatic aldehydes (1 mmol), 1,3-diones (1 mmol) and urea (1 mmol) in a round bottomed flask, was added 1-methyl-3-nitro-1H-imidazol-3-ium trinitromethanide {[MIM-NO2]C(NO2)3} as a novel nano ionic liquid catalyst (1 mol%), and the consequent mixture was firstly stirred magnetically under solvent-free conditions at room temperature. After completion of the reaction, as monitored by TLC n-hexane/ethyl acetate (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), ethyl acetate (10 mL) was added to the reaction mixture, stirred and refluxed for 10 min, and then was washed with water (10 mL) and decanted to separate the catalyst from other materials (the reaction mixture was soluble in hot ethyl acetate and NIL catalyst was soluble in water). The aqueous layer was decanted and the catalyst separated after the removal of water. The remaining catalyst was used for an alternative reaction. The solvent from the organic layer was evaporated and the crude product was purified via recrystallization from ethanol/water (10
2), ethyl acetate (10 mL) was added to the reaction mixture, stirred and refluxed for 10 min, and then was washed with water (10 mL) and decanted to separate the catalyst from other materials (the reaction mixture was soluble in hot ethyl acetate and NIL catalyst was soluble in water). The aqueous layer was decanted and the catalyst separated after the removal of water. The remaining catalyst was used for an alternative reaction. The solvent from the organic layer was evaporated and the crude product was purified via recrystallization from ethanol/water (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In this work, nano ionic liquid catalyst was recycled and reused four times without any significant loss of its catalytic activity.
1). In this work, nano ionic liquid catalyst was recycled and reused four times without any significant loss of its catalytic activity.
Spectral data for compounds
Ethyl 6-amino-4-(naphthalen-1-yl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (Table 4, entry 1): white solid; m.p: 240–242 °C; yield: 93%; IR (KBr): ν 3463, 3423, 3258, 3295, 3052, 2978, 1699, 1677, 1523, 1232 cm−1; 1H NMR (400 MHz, DMSO-d6): δppm 0.84 (t, 3H, J = 7.2 Hz, –CH3), 3.84 (q, 2H, J = 6.4 Hz, –CH2), 5.63 (s, 2H, –NH2), 6.80 (s, 1H, –CH aliphatic), 6.91 (s, 1H, –NH), 7.43 (d, 1H, J = 8.4 Hz, ArH), 7.63 (t, 2H, J = 5.4 Hz, ArH), 7.77 (s, 1H, –NH), 7.90 (t, 1H, J = 7.2 Hz, ArH), 7.98 (d, 1H, J = 8.4 Hz, ArH), 8.04 (d, 1H, J = 8.0 Hz, ArH), 8.33 (d, 1H, J = 8.0 Hz, ArH); 13C NMR (100 MHz, DMSO-d6): δppm 18.2, 50.2, 56.9, 124.6, 125.6, 126.1, 126.2, 126.5, 126.7, 128.3, 128.9, 129.0, 130.5, 130.7, 133.9, 134.0, 140.8; MS: m/z = 311 [M]+.Acknowledgements
We thank Bu-Ali Sina University and Iran National Science Foundation (INSF) for financial support (Grant No: 94002177) to our research groups.Notes and references
- D. Strubing, H. Neumann, S. Klaus, S. Hubner and M. Beller, Tetrahedron, 2005, 61, 11333 CrossRef.
- A. Davoodnia, M. M. Heravi, L. Rezaei-Daghigh and N. Tavakoli-Hoseini, Monatsh. Chem., 2009, 140, 1499 CrossRef CAS.
- L. Yu, B. Chen and X. Huang, Tetrahedron Lett., 2007, 48, 925 CrossRef CAS.
- S. V. Ryabukhin, A. S. Plaskon, E. N. Ostapchuk, D. M. Volochnyuk and A. A. Tolmachev, Synthesis, 2007, 3, 417 Search PubMed.
- P. Salehi, M. Dabiri, M. A. Zolfigol and M. A. Bodaghi, Tetrahedron Lett., 2003, 44, 2889 CrossRef CAS.
- G. C. Rovnyak, S. D. Kimball, B. Beyer, G. J. D. Cucinotta, J. Gougoutas, A. Hedberg, M. Malley, J. P. McCarthy, R. Zhang and S. Moreland, J. Med. Chem., 1995, 38, 119 CrossRef CAS PubMed.
- D. Vo, W. C. Matowe, M. Ramesh, N. Iqbal, M. W. Wolowyk, S. E. Howlett and E. E. Knaus, J. Med. Chem., 1995, 38, 2851 CrossRef CAS PubMed.
- (a) F. R. Buhler and W. Kiowski, J. Hypertens., 1987, 5, S3 CrossRef CAS; (b) M. A. Zolfigol, P. Salehi and M. Safaiee, Lett. Org. Chem., 2006, 3, 153 CrossRef CAS.
- P. Biginelli, Gazz. Chim. Ital., 1893, 23, 360 Search PubMed.
- C. O. Kappe, Tetrahedron, 1993, 49, 6937 CrossRef CAS.
- (a) J. W. Yang, M. T. H. Fonseca and B. List, Angew. Chem., Int. Ed., 2004, 43, 6660 CrossRef PubMed; (b) N. J. A. Martin and B. List, J. Am. Chem. Soc., 2006, 128, 13368 CrossRef CAS PubMed.
- S. Hoffmann, M. Nicoletti and B. List, J. Am. Chem. Soc., 2006, 128, 13074 CrossRef CAS PubMed.
- M. Rueping, A. P. Antonchick and T. Theissmann, Angew. Chem., Int. Ed., 2006, 45, 3683 CrossRef CAS PubMed.
- J. Krechl and S. Smrčková, Tetrahedron Lett., 1989, 30, 5315 CrossRef CAS.
- M. M. Heravi, F. Derikvand and F. Bamoharram, J. Mol. Catal. A: Chem., 2005, 242, 173 CrossRef CAS.
- A. Rahmatpour, Catal. Lett., 2012, 142, 1505 CrossRef CAS.
- H. R. Kalita and P. Phukan, Catal. Commun., 2007, 8, 179 CrossRef CAS.
- M. A. Bigdeli, S. Jafari, G. H. Mahdavinia and H. Hazarkhani, Catal. Commun., 2007, 8, 1641 CrossRef CAS.
- Y. Yu, D. Liu, C. Liu and G. Luo, Bioorg. Med. Chem. Lett., 2007, 17, 3508 CrossRef CAS PubMed.
- M. M. Heravi, K. Bakhtiari and F. F. Bamoharram, Catal. Commun., 2006, 7, 373 CrossRef CAS.
- F. Endres and S. Z. E. Abedin, Phys. Chem. Chem. Phys., 2006, 8, 2101 RSC.
- J. F. Wishart and E. W. Castner Jr, J. Phys. Chem. B, 2007, 111, 4639 CrossRef CAS.
- K. R. Seddon, J. Chem. Technol. Biotechnol., 1997, 68, 351 CrossRef CAS.
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinheim, 2nd edn, 2008 Search PubMed.
- C. Chiappe and D. Pieraccini, J. Phys. Org. Chem., 2005, 18, 275 CrossRef CAS.
- J. Ranke, S. Stolte, R. Stormann, J. Arning and B. Jastorff, Chem. Rev., 2007, 107, 2183 CrossRef CAS PubMed.
- M. J. Earle and K. R. Seddon, Pure Appl. Chem., 2000, 72, 1391 CrossRef CAS.
- S. G. Lee, Chem. Commun., 2006, 1049 RSC.
- R. A. Sheldon, Green Chem., 2005, 7, 267 RSC.
- (a) R. D. Rogers and K. R. Seddon, Ionic Liquids as Green Solvents. Progress and Prospects, Oxford University Press, USA, Washington, 2003 Search PubMed; (b) C. E. Song, Chem. Commun., 2004, 1033 RSC; (c) N. Jain, A. Kumar, S. Chauhan and S. M. S. Chauhan, Tetrahedron, 2005, 61, 1015 CrossRef CAS; (d) S. V. Malhotra, V. Kumar and V. S. Parmar, Curr. Org. Synth., 2007, 4, 370 CrossRef CAS; (e) J. Durand, E. Teuma and M. Gómez, C. R. Chim., 2007, 10, 152 CrossRef CAS; (f) V. I. Parvulescu and C. Hardacre, Chem. Rev., 2007, 107, 2615 CrossRef CAS PubMed; (g) N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123 RSC.
- (a) R. Sebesta, I. Kmentová and S. Toma, Green Chem., 2008, 10, 484 RSC; (b) W. Miao and T. H. Chan, Acc. Chem. Res., 2006, 39, 897 CrossRef CAS PubMed.
- M. Gruttadauria, F. Giacalone and R. Noto, Chem. Soc. Rev., 2008, 37, 1666 RSC.
- T. L. Greaves and C. J. Drummond, Chem. Rev., 2008, 108, 206 CrossRef CAS PubMed.
- (a) C. Baudequin, J. Baudoux, J. Levillain, D. Cahard, A. C. Gaumont and J. C. Plaquevent, Tetrahedron: Asymmetry, 2003, 14, 3081 CrossRef CAS; (b) C. Baudequin, D. Brégeon, J. Levillain, F. Guillen, J. C. Plaquevent and A. C. Gaumont, Tetrahedron: Asymmetry, 2005, 16, 3921 CrossRef CAS; (c) X. Chen, X. Li, A. Hu and F. Wang, Tetrahedron: Asymmetry, 2008, 19, 1 CrossRef CAS; (d) A. Winkel, P. V. Reddy and R. Wilhelm, Synthesis, 2008, 999 CAS; (e) K. Bica and P. Gaertner, Eur. J. Org. Chem., 2008, 3235 CrossRef CAS.
- M. Shiri, M. A. Zolfigol, H. G. Kruger and Z. Tanbakouchian, Tetrahedron, 2010, 66, 9077 CrossRef CAS ; and our references cited therein.
- (a) N. Iranpoor, H. Firouzabadi and M. A. Zolfigol, Synth. Commun., 1998, 28, 2773 CrossRef CAS; (b) N. Iranpoor, H. Firouzabadi and M. A. Zolfigol, Synth. Commun., 1997, 27, 1247 CrossRef CAS; (c) N. Iranpoor, H. Firouzabadi and M. A. Zolfigol, Synth. Commun., 1998, 28, 367 CrossRef CAS; (d) H. Firouzabadi, N. Iranpoor and M. A. Zolfigol, Synth. Commun., 1998, 28, 377 CrossRef CAS; (e) N. Iranpoor, H. Firouzabadi and M. A. Zolfigol, Bull. Chem. Soc. Jpn., 1998, 71, 905 CrossRef CAS; (f) H. Firouzabadi, N. Iranpoor and M. A. Zolfigol, Bull. Chem. Soc. Jpn., 1998, 71, 2169 CrossRef CAS.
- M. A. Zolfigol, M. H. Zebarjadian, G. Chehardoli, H. Keypour, S. Salehzadeh and M. Shamsipur, J. Org. Chem., 2001, 66, 3619 CrossRef CAS PubMed.
- M. A. Zolfigol, F. Afsharnaderee, S. Baghery, S. Salehzadeh and F. Maleki, RSC Adv., 2015, 5, 7555 Search PubMed ; and our references cited therein.
- (a) A. N. Dadhania, V. K. Patel and D. K. Raval, J. Braz. Chem. Soc., 2011, 22, 511 CrossRef CAS; (b) A. N. Dadhania, V. K. Patel and D. K. Raval, J. Chem. Sci., 2012, 124, 921 CrossRef CAS.
- (a) H. G. O. Alvim, T. B. de Lima, H. C. B. de Oliveira, F. C. Gozzo, J. L. de Macedo, P. V. Abdelnur, W. A. Silva and B. A. D. Neto, ACS Catal., 2013, 3, 1420 CrossRef CAS; (b) L. M. Ramos, A. Y. Ponce de Leon y Tobio, M. R. dos Santos, H. C. B. de Oliveira, A. F. Gomes, F. C. Gozzo, A. L. de Oliveira and B. A. D. Neto, J. Org. Chem., 2012, 77, 10184 CrossRef CAS PubMed; (c) L. M. Ramos, B. C. Guido, C. C. Nobrega, J. R. Correa, R. G. Silva, H. C. B. de Oliveira, A. F. Gomes, F. C. Gozzo and B. A. D. Neto, Chem.–Eur. J., 2013, 19, 4156 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra24619a |
| This journal is © The Royal Society of Chemistry 2016 |