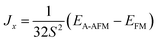First-principles study of the electronic and magnetic properties of the spin-ladder iron oxide Sr3Fe2O5
Xianfeng Hao*,
Yuanhui Xu*,
Shanshan Liu,
Jing Wang and
Faming Gao
Key Laboratory of Applied Chemistry, Department of Chemical Engineering, Yanshan University, Qinhuangdao 066004, P. R. China. E-mail: xfhao@ysu.edu.cn; yhxu@ysu.edu.cn; Fax: +86-335-8561569
First published on 12th January 2016
Abstract
The electronic and magnetic properties of the novel spin-ladder iron oxide Sr3Fe2O5, containing an unusual square-planar coordination around high-spin Fe2+ cations, were investigated using the generalized gradient approximation plus the Coulomb interaction correlation method. Our results demonstrated that the G-type antiferromagnetic configuration is the ground state, which is in excellent agreement with experimental neutron powder diffraction and Mössbauer spectroscopy measurements, as well as available theoretical results in the literature, albeit with slightly larger computed magnetic moments. Moreover, the outstanding discrepancy between the two-dimensional crystal structure and the three-dimensional electronic/magnetic properties was resolved via the special localization and orientation of electronic/spin charge in real space, i.e., the dz2 orbital ordering of the down-spin Fe 3d electron.
Introduction
The chemistry and physics of transition metal oxides have been full of surprises and intriguing structural, electronic and magnetic phenomena. One of the key ingredients of the origin of these effects is the availability of degenerate electronic orbitals. Whether such orbital degrees of freedom actually occur in a material depends on the nature of the atomic ions and the surrounding crystallographic structure, i.e., the coordination polyhedra. Thus, the ability to tune the coordination polyhedra of transition metal ions will offer an extra method of designing novel materials, which might possess fascinating properties and potential applications.The coordination geometries of iron oxides have been exclusively restricted to three-dimensional polyhedra such as octahedra and tetrahedra. However, this restriction was recently circumvented by using calcium hydride as a powerful reductant at low temperatures,1 as initiated and developed by Hayward et al., which provides an effective route for obtaining unprecedented coordination polyhedra in transition metal oxides.2 By applying this new synthetic method to SrFeO3, a new compound SrFeO2, which displays an unusual square-planar coordination around Fe, has been obtained. It contains planar FeO2 layers that comprise corner-sharing FeO4 squares with high-spin Fe2+ ions separated by Sr2+ ions,1,3 which are isostructural with the undoped high-Tc superconductor SrCuO2. Compared with compounds with low dimensionality, SrFeO2 exhibits interesting and apparently puzzling physical properties.1 From the theoretical point of view, most of the available insight that originates from electronic structure calculations4,5 indicates that the lone down-spin electron of the high-spin Fe2+ (d6) ion located in the square-planar coordinated structure occupies the dz2 orbital. This special electronic configuration indicates that SrFeO2 is not subject to Jahn–Teller distortion when the temperature is lowered, and is associated with a three-dimensional antiferromagnetic spin order with a very high Néel temperature (TN = 473 K).
The low-temperature reaction of the double-layered perovskite Sr3Fe2O7 (space group I4/mmm) with CaH2 gives rise to the stable novel spin-ladder iron oxide Sr3Fe2O5.6,7 Sr3Fe2O5 adopts an I-centered orthorhombic space group (Immm) with a = 3.51485 Å, b = 3.95271 Å and c = 20.91251 Å,6 as shown in Fig. 1. It consists of double layers of corner-sharing planar FeO2 interleaved by Sr2+ ions along the z direction, and exhibits a framework of ladders with two legs running along the [010] direction and rungs along the [001] direction. Neutron powder diffraction and Mössbauer spectroscopy measurements established the presence of long-range antiferromagnetic order, which is characterized by a magnetic propagation vector q = (1/2, 1/2, 0), and that the moments of iron of 2.76 μB are aligned parallel to the z direction.6,7 In addition, first-principles density functional theory calculations employing the projected augmented wave method suggested that magnetic dipole–dipole interactions are essential for the three-dimensional magnetic ordering in Sr3Fe2O5.8 Furthermore, identifying the fine details of the electronic structure and its tendency towards a magnetic configuration, as well as the strength of the coupling of the magnetic ions, is important for understanding the physical properties. Generally, it is well known that electronic and magnetic properties are very sensitive to coordination polyhedra. To the best of our knowledge, a clear picture of the role of the unusual square-planar coordination of Fe2+ ions in Sr3Fe2O5 is lacking and deserves special attention. In an attempt to partly remedy this situation, we have investigated the electronic and magnetic properties of Sr3Fe2O5 using full-potential density functional theory calculations. The implications of our work in relation to recent experiments are discussed in detail.
 | ||
| Fig. 1 The spin structure of Sr3Fe2O5 below TN. The large green and small red spheres represent Sr and O atoms, respectively. The arrows indicate the direction of the moment on the iron ions. | ||
Approach
The band structure calculation was carried out within the framework of the generalized gradient approximation (GGA) + U method.9–12 We used the highly accurate WIEN2k package,13,14 which implements the linear augmented plane wave (LAPW) method with local orbitals.15,16 In this method, the wavefunctions are expanded in spherical harmonics inside non-overlapping atomic spheres of a radius of RMT and in plane waves in the interstitial region. We adopted the experimentally determined crystal structure with the space group Immm. In the calculation, we chose values of RMT of 2.45, 1.95 and 1.73 Bohr for Sr, Fe and O, respectively. The Ewald cutoff radius was RMTKmax = 7. The maximum value of l for the expansion of the wavefunctions in spherical harmonics inside the spheres was taken to be lmax = 10. The total number of k-points was 1000 in the whole Brillouin zone.17 To account for the strong electron correlation associated with the Fe 3d states, GGA + U calculations with an effective value of Ueff = 4.0 eV were performed; this value is similar to that in previous studies of Sr3Fe2O5 (ref. 8) and SrFeO2.4,5,18 To test the sensitivity of our results to the on-site effective Coulomb repulsion, somewhat larger and smaller values of Ueff were also used for the Fe d electrons; our conclusion still holds, given that a sizable value of Ueff is present at the Fe2+ sites. The self-consistent calculations were considered to be converged only when the integrated charge difference per formula unit between input charge densities was less than 0.0001.Results and discussion
In order to determine the magnetic ground state of Sr3Fe2O5, three spin ordering configurations were studied, namely, a ferromagnetic arrangement, an A-type antiferromagnetic arrangement along the x direction, in which ferromagnetically ordered planes of Fe sites are antiferromagnetically coupled within each plane, and a G-type antiferromagnetic arrangement, in which antiferromagnetically ordered planes of Fe sites are antiferromagnetically coupled. The results of our calculations for these three magnetic configurations of the orthorhombic phase of Sr3Fe2O5 are summarized in Table 1. The G-type antiferromagnetic state possesses the lowest energy, which is consistent with experimental determinations based on neutron powder diffraction,6,7 irrespective of the calculation methods (GGA or GGA + U), and in fair agreement with previous theoretical reports.8 Concerning the magnetic ground state configuration, we determined the value of the superexchange parameters in terms of the classic Heisenberg spin Hamiltonian:| FM | A-AFM | G-AFM | ||
|---|---|---|---|---|
| Total energy | GGA(U = 0.0 eV) | 0.095 eV per a.u. | 0.098 eV per a.u. | 0.0 eV per a.u. |
| GGA + U(U = 4.0 eV) | 0.08718 eV per a.u. | 0.03773 eV per a.u. | 0.0 eV per a.u. | |
| Fe | O | Fe | O | Fe | O | ||
|---|---|---|---|---|---|---|---|
| Magnetic moment | GGA(U = 0.0 eV) | 3.19 | 0.09–0.17 | ±3.36 | ±0.11–±0.19 | ±3.30 | ±0.10 |
| GGA + U(U = 4.0 eV) | 3.57 | 0.08–0.12 | ±3.55 | ±0.07–±0.11 | ±3.54 | ±0.07 | |
Here, J > 0 (J < 0) represents ferromagnetic (antiferromagnetic) interactions and S = 2 is the spin of 3d6 Fe2+ ions. On the basis of our calculations, we only considered the Jyz interaction within the yz planes and the Jx interaction between the yz planes along the x direction, respectively. The interaction between the perovskite building blocks was omitted, owing to the large distances.
According to the GGA + U(Ueff = 4.0 eV) results, we obtained values of Jyz = −1.57 meV and Jx = −3.10 meV. Note that both interactions are negative, which indicates antiferromagnetic interactions within the two-dimensional planes and between them, which confirms that the ground state is G-type antiferromagnetic. Owing to the two-dimensional character of the structure, however, one could expect that the strength of interplanar coupling should be small, exhibiting two-dimensional magnetic properties. Unexpectedly, it was found that the robust three-dimensional G-type antiferromagnetic state within the double layers of corner-sharing planar FeO2 is the ground state as stated above, which is in apparent contrast to expectations. This suggests that the electronic configuration of Fe2+ within the planar coordination plays an important role in the magnetic properties of Sr3Fe2O5.
The calculated magnetic moment at the Fe sites varies between 3.19 μB and 3.30 μB per Fe atom depending upon the magnetic configuration considered in our calculations at the GGA level. The calculated magnetic moment at the Fe sites was found to be slightly larger than the value of 2.76 μB measured in low-temperature neutron diffraction measurements,6,7 whereas it is substantially smaller than the expected value. One of the reasons for the large decrease is that the effect of quantum magnetic fluctuations is inherently enhanced in the low-dimensional ladder, as was argued in ref. 6. Alternatively, the calculated magnetic moment at the iron sites might be reduced from the expected integer value of 4 μB, because the Fe d electrons exhibit hybridization interactions with the neighboring O ions. Owing to the hybridization interactions, we found that a magnetic moment of around 0.10 μB was induced at each O site, whereas the direction of the polarization depended on the magnetic configuration. The inclusion of an on-site Coulomb correlation on the Fe 3d electrons (Ueff = 4.0 eV) under the GGA + U formulation increases the computed magnetic moments. The remarkable increase in the theoretical value at the Fe sites is accompanied by a slight decrease in the computed value at the O sites. These observations are expected, because the GGA + U scheme tends to enhance the “localization” of Fe d states and then diminish the hybridization interactions between the Fe d electrons and the neighboring O ions.
Because the GGA + U method yielded a similar density of states and charge density to the GGA approach, the results discussed as follows are restricted to the GGA scheme unless otherwise indicated. Note that the ferromagnetic configuration (which is experimentally inaccessible) might provide useful references for understanding the G-type antiferromagnetic configuration that was confirmed. Fig. 2 shows the total and partial density of states corresponding to the Fe 3d electronic states of the ferromagnetic configuration. Majority and minority spins are shown above and below the vertical axis, respectively. The plots clearly demonstrate that there is a strong hybridization interaction between the Fe 3d states and the surrounding O 2p states. Moreover, one can observe that the Fe 3d spin-up states are completely occupied, whereas the spin-down states are partially occupied and cross the Fermi level. This reflects the large exchange splitting of the Fe 3d levels, which is responsible for the high-spin state of the Fe2+ ions and the computed magnetic moments. As a consequence, the GGA calculations for the ferromagnetic configuration converged to the metallic ground state with a high density of states at the Fermi level, which indicates the instability of this arrangement.
 | ||
| Fig. 2 Total (black solid line) and partial density of states of Fe (red dashed/dotted line) for the ferromagnetic configuration of Sr3Fe2O5 obtained via GGA calculations. | ||
However, when one introduces G-type antiferromagnetic ordering into the framework, the exchange interaction produces an exchange potential that effectively shifts the energy of the Fe 3d states to lower energy, giving rise to insulating behavior. The calculated band structures along the high-symmetry directions of the first Brillouin zone, as well as the total density of states, are presented in Fig. 3 and Fig. 4, respectively. It is evident that the antiferromagnetic coupling leads to a significant reduction in the bandwidth of the energy bands and a redistribution in spectral weight. A closer inspection of the representations of the site-projected density of states reveals that the Sr atoms have largely donated their valence electrons to the other constituents and have themselves entered into nearly pure ionic states (which is evident from the small number of states in the valence band and the larger number of states in the conduction band). Because the Fe and O states cover the same energy range with a similar pattern of the density of states curve, to some extent, there may be appreciable hybridization, that is, a significant degree of Fe–O covalent bonding character. More importantly, a thorough inspection of the density of states reveals that the electronic configuration is (dz2)2(dxzdyz)2(dxy)1(dx2−y2)1 for the Fe2+ cations, where the minority-spin electron exclusively occupies the dz2 orbital, free from Jahn–Teller distortion. This scenario is in accordance with the experimental observations6,7 and the existing theoretical results.8,19 Taking the Coulomb correlation effects into account, although the band gap of Sr3Fe2O5 for the G-type AFM configuration increases from 0.6 eV to 1.5 eV, the pattern of the density of states remains intact. In particular, the dispersion of the energy band along all the highly symmetrical directions of the Brillouin zone is moderate and isotropic, demonstrating strong three-dimensional behavior of the electronic properties, which is in sharp contrast with the two-dimensional crystallographic structure of Sr3Fe2O5.
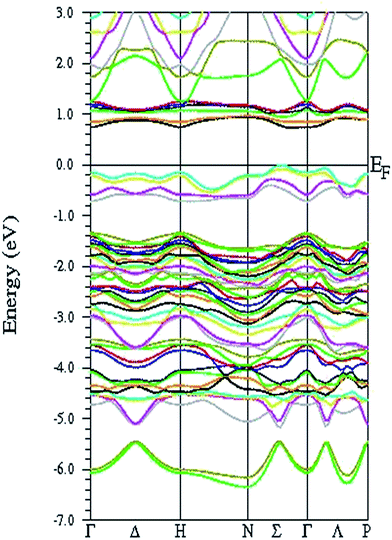 | ||
| Fig. 3 Band structure for G-type antiferromagnetic configuration of Sr3Fe2O5 obtained via GGA calculations. The Fermi level, EF, is set to 0 eV. | ||
 | ||
| Fig. 4 Total density of states for the G-type antiferromagnetic configuration of Sr3Fe2O5 obtained via GGA calculations. | ||
Comparing the three-dimensional electronic/magnetic properties with the two-dimensional crystallographic structure, these features pose the interesting question of how this conundrum could be reconciled. Elucidating this paradox will no doubt lead to an improvement in our understanding of the diverse behavior of transition metal oxides, because of their versatile charge, spin and orbital degrees of freedom.
To reveal the mechanism behind the interesting and puzzling physical properties of Sr3Fe2O5, we turned our attention to the spin density distribution of the G-type antiferromagnetic configuration of Sr3Fe2O5 by plotting the local spin-up/down electron density for the energy window of 0.6 eV just below EF, as illustrated in Fig. 5. The occupied majority/minority spin state of the sixth 3d electron is predominantly of dz2 orbital character, rather than the doubly degenerate dxz and dyz orbitals. Moreover, such localization in real space is accompanied by flat band levels in the Brillouin zone. Such a special electronic state originates from what is expected from the crystal field splitting of the D4h point symmetry and molecular orbital energy,18 or the strong hybridization of the iron 3dz2 and 4s orbitals, as proposed by Takano et al.5 Hence, the three-dimensional G-type antiferromagnetic configuration could be explained on the basis of superexchange coupling and the special localization and orientation of electronic/spin charge in real space. On the one hand, according to the Goodenough–Kanamori–Anderson (GKA) rules,20 it is well established that the spins of the Fe cations within the yz plane (Fig. 1) are aligned antiferromagnetically owing to linear Fe–O–Fe superexchange interactions. On the other hand, the obtained pattern of ferro-orbital order induces antiferromagnetic coupling along the x axis between the interplanes, which is in close accordance with the Kugel–Khomskii model.21 To sum up, we addressed the origin of the unusual and puzzling physical properties of Sr3Fe2O5 and found that it might be associated with the dz2 orbital ordering of the minority-spin 3d electron. This accounts for the modest G-type antiferromagnetic configuration and in turn the corresponding three-dimensional electronic properties.
Conclusions
In conclusion, we have investigated the electronic and magnetic properties of Sr3Fe2O5, which is an unusual spin-ladder iron oxide with square-planar coordination around high-spin Fe2+. The obtained results demonstrated that the G-type antiferromagnetic configuration is the ground state, which is in excellent agreement with experimental neutron powder diffraction and Mössbauer spectroscopy measurements and existing theoretical reports, despite the slightly larger calculated magnetic moments. The paradox that the two-dimensional square lattice exhibits modest three-dimensional electronic and magnetic properties could be understood through the dz2 orbital ordering of the sixth minority 3d electron, on the basis of superexchange interactions and the ferro-orbital ordering pattern. The special localization and orientation of electronic/spin charge in real space is the key factor for understanding the structural stability and electronic and magnetic properties of the material.Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 21201148, 21303156, 21403185, and 21543006), Natural Science Foundation of Hebei province (Grant No. B2015203105), and Postdoctoral Science Foundation of China No. 2015M571277 and 2014M551048.References
- Y. Tsujimoto, C. Tassel, N. Hayashi, T. Watanabe, H. Kageyama, K. Yoshimura, M. Takano, M. Ceretti, C. Ritter and W. Paulus, Nature, 2007, 450, 1062 CrossRef CAS PubMed.
- (a) M. A. Hayward, M. A. Green, M. J. Rosseinsky and J. Sloan, J. Am. Chem. Soc., 1999, 121, 8843 CrossRef CAS; (b) M. A. Hayward, E. J. Cussen, J. B. Claridge, M. Bieringer, M. J. Rosseinsky, C. J. Kiely, S. J. Blundell, I. M. Marshall and F. L. Pratt, Science, 2002, 295, 1882 CrossRef CAS PubMed.
- C. Tasssel, T. Watanabe, Y. Tsujimoto, N. Hayashi, A. Kitada, Y. Sumida, T. Yamamoto, H. Kageyama, M. Takano and K. Yoshimura, J. Am. Chem. Soc., 2008, 130, 3764 CrossRef PubMed.
- H. J. Xiang, S.-H. Wei and M.-H. Whangbo, Phys. Rev. Lett., 2008, 100, 167207 CrossRef CAS PubMed.
- J. M. Pruneda, J. Íñiguez, E. Canadell, H. Kageyama and M. Takano, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 115101 CrossRef.
- H. Kageyama, T. Watanabe, Y. Tsujimoto, A. Kitada, Y. Sumida, K. Kanamori, K. Yoshimura, N. Hayashi, S. Muranaka, M. Takano, M. Ceretti, W. Paulus, C. Retter and G. André, Angew. Chem., Int. Ed., 2008, 47, 5740 CrossRef CAS PubMed.
- T. Yamamoto, C. Tassel, Y. Kobayashi, T. Kawakami, T. Okada, T. Yagi, H. Yoshida, T. Kamatani, Y. Watanabe, T. Kikegawa, M. Takano, K. Yoshimura and H. Kageyama, J. Am. Chem. Soc., 2011, 133, 6036 CrossRef CAS PubMed.
- H.-J. Koo, H. Xiang, C. Lee and M.-H. Whangbo, Inorg. Chem., 2009, 48, 9051 CrossRef CAS PubMed.
- J. Perdew, S. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- A. Lichtenstein, V. Anisimov and J. Zaanen, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 52, R5467 CrossRef.
- V. Anisimov, F. Aryasetiawan and A. Lichtenstein, J. Phys.: Condens. Matter, 1997, 9, 767 CrossRef CAS.
- A. Petukhov, I. Mazin, L. Chioncel and A. Lichtenstein, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 153106 CrossRef.
- K. Schwarz and P. Blaha, Comput. Mater. Sci., 2003, 28, 259 CrossRef CAS.
- P. Blaha, K. Schwarz, G. K. H. Madsen, D. Kvasnicka and J. Luitz, WIEN2k, An Augmented Plane Wave Plus Local Orbitals Program for Calculating Crystal Properties, Vienna University of Technology, Austria, 2001, ISBN 3-9501031-1-2 Search PubMed.
- E. Sjöstedt, L. Nordström and D. J. Singh, Solid State Commun., 2000, 114, 15 CrossRef.
- G. K. H. Madsen, P. Blaha, K. Schwarz, E. Sjöstedt and L. Nordström, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 195134 CrossRef.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953 CrossRef.
- R. Mavlanjan, N. Yao-zhuang and G. Cuang-hua, Inorg. Chem., 2013, 52, 12519 Search PubMed.
- T. Jia, H. Wu, X. Zhang, T. Liu, Z. Zeng and H. Lin, EPL, 2013, 102, 67004 CrossRef.
- J. B. Goodenough, Magnetism and the Chemical Bond, Interscience-Wiley, New York, 1963; CrossRef CAS; J. Kanamori, J. Phys. Chem. Solids, 1959, 10, 87 CrossRef CAS.
- K. I. Kugel and D. I. Khomskii, Sov. Phys. Solid State, 1975, 17, 285 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |