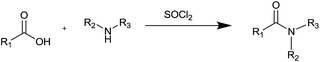
One-pot synthesis of amides from carboxylic acids activated using thionyl chloride†
A. Leggio*a,
E. L. Belsitoa,
G. De Lucab,
M. L. Di Gioiaa,
V. Leottaa,
E. Romioa,
C. Sicilianoa and
A. Liguori*a
aDipartimento di Farmacia e Scienze della Salute e della Nutrizione, Università della Calabria Edificio Polifunzionale, I-87036 Arcavacata di Rende, CS, Italy. E-mail: angelo.liguori@unical.it; antonella.leggio@unical.it; Fax: +39 0984 493265; Tel: +39 0984 493205 Tel: +39 0984 493199
bDipartimento di Chimica e Tecnologie Chimiche, Università della Calabria, Via P. Bucci, I-87036 Arcavacata di Rende, Italy
First published on 1st April 2016
Abstract
A one-pot synthesis of secondary and tertiary amides from carboxylic acids and amines by using SOCl2 has been developed. Also when sterically hindered amines were used as the starting materials, excellent yields of the corresponding amides were obtained. The amidation of N-protected α-amino acids with secondary amines proceeds effectively with good yields. The process works well also in the presence of acid sensitive groups and occurs with almost complete retention of stereochemical integrity of chiral substrates. This protocol could be extended to industrial large-scale production processes.
Introduction
Amides represent one of the most important and valuable organic functional groups in naturally occurring molecules, pharmaceuticals, agrochemicals, and polymers.1The synthesis of amide is of huge importance in organic, coordination, and medicinal chemistry.2
Improved and innovative methods for the synthesis of amides are in great demand both by chemical and pharmaceutical industry.3–5
Carboxylic amides are typically obtained from amines and activated carboxylic acid derivatives through a nucleophilic acyl substitution reaction. Acid derivatives most frequently used in the amides synthesis are acyl chlorides.
The amidation of carboxylic acids via acyl chlorides is usually a two-step process,6 involving first the conversion of the acid into the acyl chloride followed by the coupling itself with the amine.
Chlorination of carboxylic acids is carried out using several chlorinating reagents, such as pivaloyl chloride, phthaloyl dichloride, thionyl chloride and oxalyl chloride.7–10
Other chlorinating reagents11 that can be employed in amide formation are 2,4,6-trichloro-1,3,5-triazine (CC, cyanuric chloride),12 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT)13 and tetramethyl-α-chloroenamine14 which gives rise to neutral side-products and bis(trichloromethyl)carbonate (BTC).15
Thionyl chloride (SOCl2) is the most popular reagent to activate carboxylic function because it is volatile and the excess can be removed easily by distillation, finally it is non-expensive.
Usually, for the preparation of acid chlorides, thionyl chloride is used, neat or dissolved in a solvent, in the presence of the corresponding acid. The reaction requires heating and can be accelerated by adding pyridine.16 Improvements of the classical SOCl2–pyridine method was achieved by forming the acid chlorides from dicyclohexylammonium salts of carboxylic acids and thionyl chloride.17
Secondary and tertiary amides were also obtained in a one pot reaction by treating carboxylic acids with thionyl chloride and stoichiometric amounts of amines in the absence of base and in the presence of N,N-disubstituted amides ((N,N-dimethylacetamide (DMAC), N,N-dimethylformamide (DMF), N-methylpyrrolidone (NMP)).18–24 The reaction occurs by generating the Vilsmeier complex from N,N-disubstituted amides (DMAC, DMF, NMP) and thionyl chloride that acts as reagent for chlorination of carboxylic acids. The formation of amides is completed in more than 1 hour and in some cases, to achieve 100% conversion, it is necessary to heat to 50 °C.18 In other cases instead, the reaction takes places in longer times and with low yields.20,21
In Table 1 is reported a comparison of different one pot thionyl chloride methods for amide synthesis.
| Entry | Reaction conditions | SOCl2 (equiv.) | Yield (%) | Time | Ref. |
|---|---|---|---|---|---|
| a Reaction solvent.b 0.05 equiv.c n.r. = not reported. | |||||
| 1 | DMACa, 20 to 50 °C | 1.08 | 80–90 | >1 h | 18 |
| 2 | DMACa, 15 to 20 °C | n.r. | 92 | n.r.c | 19 |
| 3 | DMACa, −12 to 20 °C | 1.27 | 45 | 9 h | 20 |
| 4 | DMACa, −20 to 20 °C | 1.04 | 51 | 3.5 h | 21 |
| 5 | NMPa, 0 to 25 °C | 1.04 | 85 | 1.5 h | 22 |
| 6 | DMFb, Et3N, 45 °C | 1.05 | 69 | 3 h | 23 |
| 7 | Toluenea, 40–70 °C | 1.20 | n.r. | 2–24 h | 25 |
As an alternative, the carboxyl function activation can be performed by using coupling agents26 that are also widely used for the formation of peptide bond.27–29 They allow to overcome side reactions such as ketene formation, acyl transfer and to reduce racemization in peptide coupling.
Here we report the use of thionyl chloride as activating agent of carboxylic function for the one-pot synthesis of amides.
The thionyl chloride (SOCl2), used as a chlorinating agent in the formation of acyl chlorides, could also function as coupling agent by activating in situ the carboxylic function of the acid. Then the amine present in the reaction medium could react with the reactive intermediate by generating the amide, and as a result, the reaction would proceed under one-pot conditions.
Experimental
Materials and methods
Reagents were commercially available with analytical grade and used as purchased without further purification. All α-amino acids were of the L-series. Solvents were purified according to well-known laboratory methods and freshly distilled prior to use. All reactions were carried out using flame-dried glassware and under an inert atmosphere (dry N2). Reaction mixtures were monitored by thin layer chromatography (TLC) using silica gel 60-F254 pre-coated glass plates. Spots on the TLC plates were visualized with a UV lamp (254 nm) and by spraying with 0.2% ninhydrin in ethanol and charring after elution. 1H and 13C NMR spectra were recorded at 300 MHz and 75 MHz, respectively. Spectroscopic analysis was performed at 293 K on diluted solutions of each compound by using CDCl3 as the solvent. Chemical shifts (δ) are reported in ppm and referenced to CDCl3 (singlet at 7.25 ppm for 1H and 77.0 ppm, central line of the triplet, for 13C spectra). Coupling constants (J) are reported in hertz (Hz).The 1H, 13C spectra of compounds 18, 19 and 20 were recorded at 298 k on a Bruker Avance 500 MHz instrument (1H: 500.13 MHz, 13C: 125.77 MHz).
GC-MS analyses were performed using a 30 m × 0.25 mm, PhMesiloxane capillary column. The mass detector was operated in the electron impact ionization mode (EIMS) with an electron energy of 70 eV. The injection port was heated to 250 °C. The oven temperature program was initially set at 100 °C with a hold of 2 min and ramped to 280 °C at 14 °C min−1 with a hold of 10 min.
Chiral GC-MS analyses of enantiomeric compounds 18 and 19 were performed by using a 25 m × 0.25 mm, diethyl tert-butyldimethylisilyl-β-cyclodextrine chiral capillary column. The injection port was heated to 250 °C. The oven temperature program was initially set at 50 °C ramped to 200 °C at 0.5 °C min−1 with a hold of 20 min.
ESI-QTOF mass spectra were recorded on a Quadrupole Time of Flight (QTOF) mass spectrometer fitted with an electrospray ionization source (ESI) operating in positive ion mode.
Analytical RP-HPLC analyses were carried out using a C18 RP analytical column (Tecnocroma Tracer Excel 120, ODSA, 15 × 0.4 cm, 5 μm) with an elution gradient from 20% to 40% of B over 10 minutes, from 40% to 50% of B in 20 minutes, followed by 50–60% of B in 10 minutes, A being H2O and B CH3CN and detection at 254 nm. Flow rate was 1 mL min−1.
General procedure for one pot synthesis of amides 1–19
1 mmol of carboxylic acid is added to 1 mmol of amine and 3 mmol of triethylamine (Et3N) in dichloromethane, then 1 mmol of SOCl2 is added at room temperature. The mixture is stirred for 5–20 minutes at room temperature. The recovery of the reaction product is performed by evaporating the solvent under reduced pressure. The resulting residue is taken up in dichloromethane and washed first with 1 N HCl* and then with 1 N NaOH. The organic phase was dried (Na2SO4), and evaporated to dryness to afford the corresponding carboxylic amide.*For the synthesis of N,N-diethylamides of N-Boc-protected amino acids (15, 18 and 19) the acidic work up was performed by using 5% aqueous NaHSO4.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v): 1H NMR (300 MHz, CDCl3) δ: 7.43–7.34 (m, 5H, ArH), 3.63–3.43 (m, 2H, NCH2), 3.38–3.18 (m, 2H, NCH2), 1.32–1.18 (m, 3H, CH2C
30 v/v): 1H NMR (300 MHz, CDCl3) δ: 7.43–7.34 (m, 5H, ArH), 3.63–3.43 (m, 2H, NCH2), 3.38–3.18 (m, 2H, NCH2), 1.32–1.18 (m, 3H, CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3) 1.17–1.02 (m, 3H, CH2C
3) 1.17–1.02 (m, 3H, CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (75 MHz, CDCl3) δ: 171.3, 137.2, 129.1, 128.4, 126.2, 43.3, 39.2, 14.2, 12.9. MS (EI, 70 eV) m/z (% rel.): 177 [M+˙] (20), 176 (49), 162 (8), 148 (16), 105 (100), 77 (48), 51 (21). Anal. calcd for C11H15NO: C, 74.54; H, 8.53; N, 7.90. Found: C, 74.65; H, 8.51; N, 7.88.
3); 13C NMR (75 MHz, CDCl3) δ: 171.3, 137.2, 129.1, 128.4, 126.2, 43.3, 39.2, 14.2, 12.9. MS (EI, 70 eV) m/z (% rel.): 177 [M+˙] (20), 176 (49), 162 (8), 148 (16), 105 (100), 77 (48), 51 (21). Anal. calcd for C11H15NO: C, 74.54; H, 8.53; N, 7.90. Found: C, 74.65; H, 8.51; N, 7.88.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v): Rf = 0.39; 1H NMR (300 MHz, CDCl3) δ: 8.83–8.78 (m, 1H, ArH), 8.56–8.50 (m, 1H, ArH), 8.49–8.43 (m, 1H, ArH), 3.49 (q, J = 6.5 Hz, 2H, NCH2), 3.31 (q, J = 6.5 Hz, 2H, NCH2), 1.19 (t, J = 6.5 Hz, 3H, CH2C
30 v/v): Rf = 0.39; 1H NMR (300 MHz, CDCl3) δ: 8.83–8.78 (m, 1H, ArH), 8.56–8.50 (m, 1H, ArH), 8.49–8.43 (m, 1H, ArH), 3.49 (q, J = 6.5 Hz, 2H, NCH2), 3.31 (q, J = 6.5 Hz, 2H, NCH2), 1.19 (t, J = 6.5 Hz, 3H, CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.08 (t, J = 6.5 Hz, 3H, CH2C
3), 1.08 (t, J = 6.5 Hz, 3H, CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (75 MHz, CDCl3) δ: 166.1, 150.2, 144.9, 144.7, 142.5, 43.2, 40.4, 14.2, 12.6. GC/MS: m/z 179 [M+˙] (5), 107 (30), 79 (35), 72 (100). Anal. calcd for C9H13N3O: C, 60.32; H, 7.31; N, 23.45. Found: C, 60.40; H, 7.29; N, 23.51.
3); 13C NMR (75 MHz, CDCl3) δ: 166.1, 150.2, 144.9, 144.7, 142.5, 43.2, 40.4, 14.2, 12.6. GC/MS: m/z 179 [M+˙] (5), 107 (30), 79 (35), 72 (100). Anal. calcd for C9H13N3O: C, 60.32; H, 7.31; N, 23.45. Found: C, 60.40; H, 7.29; N, 23.51.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v): Rf = 0.75; 1H NMR (300 MHz, CDCl3) δ: 7.50–7.23 (m, 9H, ArH), 3.85–3.70 (m, 1H, NCH2), 3.10–2.89 (m, 2H, NCH2), 2.71–2.52 (m, 1H, NCH2), 0.89 (t, J = 6.8 Hz, 3H, CH2C
30 v/v): Rf = 0.75; 1H NMR (300 MHz, CDCl3) δ: 7.50–7.23 (m, 9H, ArH), 3.85–3.70 (m, 1H, NCH2), 3.10–2.89 (m, 2H, NCH2), 2.71–2.52 (m, 1H, NCH2), 0.89 (t, J = 6.8 Hz, 3H, CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 0.74 (t, J = 6.8 Hz, 3H, CH2C
3), 0.74 (t, J = 6.8 Hz, 3H, CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (75 MHz, CDCl3) δ: 170.7, 139.7, 138.4, 136.1, 128.9, 128.6, 128.5, 128.3, 127.5, 126.8, 42.3, 38.4, 13.3, 11.9; MS (EI, 70 eV) m/z (% rel.): 253 [M+˙] (37), 252 (75), 181 (100), 152 (51), 77 (38). Anal. calcd for C17H19NO: C, 80.60; H, 7.56; N, 5.53. Found: C, 80.72; H, 7.58; N, 5.54.
3); 13C NMR (75 MHz, CDCl3) δ: 170.7, 139.7, 138.4, 136.1, 128.9, 128.6, 128.5, 128.3, 127.5, 126.8, 42.3, 38.4, 13.3, 11.9; MS (EI, 70 eV) m/z (% rel.): 253 [M+˙] (37), 252 (75), 181 (100), 152 (51), 77 (38). Anal. calcd for C17H19NO: C, 80.60; H, 7.56; N, 5.53. Found: C, 80.72; H, 7.58; N, 5.54.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v): Rf = 0.55; 1H NMR (300 MHz, CDCl3) δ: 7.73 (d, J = 15.3 Hz, 1H, CH vinyl), 7.61–7.50 (m, 2H, ArH), 7.40–7.34 (m. 3H, ArH), 6.84 (d, J = 15.3 Hz, 1H, CH vinyl), 3.60–3.40 (m, 4H, NCH2), 1.33–1.15 (m, 6H, CH2C
30 v/v): Rf = 0.55; 1H NMR (300 MHz, CDCl3) δ: 7.73 (d, J = 15.3 Hz, 1H, CH vinyl), 7.61–7.50 (m, 2H, ArH), 7.40–7.34 (m. 3H, ArH), 6.84 (d, J = 15.3 Hz, 1H, CH vinyl), 3.60–3.40 (m, 4H, NCH2), 1.33–1.15 (m, 6H, CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3) ppm; 13C NMR (75 MHz, CDCl3) δ: 165.7, 142.3, 135.5, 129.4, 128.7, 127.7, 117.7, 42.3, 41.1, 15.1, 13.2 ppm; MS (EI, 70 eV) m/z (% rel.): 203 [M+˙] (31), 188 (20), 131 (100), 103 (40), 77 (23). Anal. calcd for C13H17NO: C, 76.81; H, 8.43; N, 6.89. Found: C, 76.76; H, 8.41; N, 6.90.
3) ppm; 13C NMR (75 MHz, CDCl3) δ: 165.7, 142.3, 135.5, 129.4, 128.7, 127.7, 117.7, 42.3, 41.1, 15.1, 13.2 ppm; MS (EI, 70 eV) m/z (% rel.): 203 [M+˙] (31), 188 (20), 131 (100), 103 (40), 77 (23). Anal. calcd for C13H17NO: C, 76.81; H, 8.43; N, 6.89. Found: C, 76.76; H, 8.41; N, 6.90.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v): Rf = 0.68; 1H NMR (300 MHz, CDCl3) δ: 3.42–3.20 (m, 4H, NCH2), 2.35 (t, J = 7.2 Hz, 2H, CH2CO), 1.67–1.50 (m, 2H, C
30 v/v): Rf = 0.68; 1H NMR (300 MHz, CDCl3) δ: 3.42–3.20 (m, 4H, NCH2), 2.35 (t, J = 7.2 Hz, 2H, CH2CO), 1.67–1.50 (m, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH2CO), 1.34–1.10 (m, 30H, CH3(C
2CH2CO), 1.34–1.10 (m, 30H, CH3(C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2)12CH2CH2CO and (C
2)12CH2CH2CO and (C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3CH2)2N), 0.81 (t, J = 6.6 Hz, 3H, (CH2)14C
3CH2)2N), 0.81 (t, J = 6.6 Hz, 3H, (CH2)14C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (75 MHz, CDCl3) δ: 173.8, 34.2, 32.5, 31.8, 29.6, 29.4, 29.3, 29.2, 25.7, 24.8, 22.6, 14.0; MS (EI, 70 eV) m/z (% rel.): 311 [M+˙] (12), 128 (25), 115 (100), 100 (28), 72 (16). Anal. calcd for C20H41NO: C, 77.10; H, 13.26; N, 4.50. Found: C, 77.18; H, 13.29; N, 4.48.
3); 13C NMR (75 MHz, CDCl3) δ: 173.8, 34.2, 32.5, 31.8, 29.6, 29.4, 29.3, 29.2, 25.7, 24.8, 22.6, 14.0; MS (EI, 70 eV) m/z (% rel.): 311 [M+˙] (12), 128 (25), 115 (100), 100 (28), 72 (16). Anal. calcd for C20H41NO: C, 77.10; H, 13.26; N, 4.50. Found: C, 77.18; H, 13.29; N, 4.48.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v): Rf = 0.67, 1H NMR (300 MHz, CDCl3) δ: 3.38 (q, J = 7.2 Hz, 2H, NCH2), 3.31 (q, J = 7.2 Hz, 2H, NCH2), 2.29 (t, J = 7.2 Hz, 2H, CH2CO), 1.70–1.59 (m, 4H, (C
30 v/v): Rf = 0.67, 1H NMR (300 MHz, CDCl3) δ: 3.38 (q, J = 7.2 Hz, 2H, NCH2), 3.31 (q, J = 7.2 Hz, 2H, NCH2), 2.29 (t, J = 7.2 Hz, 2H, CH2CO), 1.70–1.59 (m, 4H, (C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2)2CH2CO), 1.38–1.23 (m, 18H, CH3(C
2)2CH2CO), 1.38–1.23 (m, 18H, CH3(C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2)9(CH2)2CH2CO), 1.18 (t, J = 7.2 Hz, 3H, CH2C
2)9(CH2)2CH2CO), 1.18 (t, J = 7.2 Hz, 3H, CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.12 (t, J = 7.2 Hz, 3H, NCH2C
3), 1.12 (t, J = 7.2 Hz, 3H, NCH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 0.89 (t, J = 6.6 Hz, 3H, C
3), 0.89 (t, J = 6.6 Hz, 3H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3(CH2)12CO); 13C NMR (75 MHz, CDCl3) δ: 172.2, 41.9, 39.9, 33.1, 31.9, 29.6, 29.5, 29.4, 29.3, 25.5, 22.6, 14.4, 14.1, 13.1; MS (EI, 70 eV) m/z (% rel.): 283 [M+˙] (12), 128 (25), 115 (100), 100 (28), 72 (16). Anal. calcd for C18H37NO: C, 76.26; H, 13.16; N, 4.94. Found: C, 76.37; H, 13.27; N, 4.95.
3(CH2)12CO); 13C NMR (75 MHz, CDCl3) δ: 172.2, 41.9, 39.9, 33.1, 31.9, 29.6, 29.5, 29.4, 29.3, 25.5, 22.6, 14.4, 14.1, 13.1; MS (EI, 70 eV) m/z (% rel.): 283 [M+˙] (12), 128 (25), 115 (100), 100 (28), 72 (16). Anal. calcd for C18H37NO: C, 76.26; H, 13.16; N, 4.94. Found: C, 76.37; H, 13.27; N, 4.95.![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3)2 and CH2C
3)2 and CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (75 MHz, CDCl3) δ: 171.1, 129.9, 128.9, 128.4, 125.9, 50.2, 35.2, 21.1, 14.8; m/z (% rel.): [M+˙] 191 (22), 190 (25), 176 (5), 162 (10), 148 (5), 105 (100), 77 (35). Anal. calcd for C12H17NO: C, 75.35; H, 8.96; N, 7.32. Found: C, 75.48; H, 8.94; N, 7.31.
3); 13C NMR (75 MHz, CDCl3) δ: 171.1, 129.9, 128.9, 128.4, 125.9, 50.2, 35.2, 21.1, 14.8; m/z (% rel.): [M+˙] 191 (22), 190 (25), 176 (5), 162 (10), 148 (5), 105 (100), 77 (35). Anal. calcd for C12H17NO: C, 75.35; H, 8.96; N, 7.32. Found: C, 75.48; H, 8.94; N, 7.31.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v): Rf = 0.50; 1H NMR (300 MHz, CDCl3) δ: 7.29–7.15 (m, 5H, ArH), 3.65–3.45 (m, 2H, NCH2), 3.28–3.10 (m, 2H, NCH2), 1.58–1.27 (m, 6H, NCH2(C
30 v/v): Rf = 0.50; 1H NMR (300 MHz, CDCl3) δ: 7.29–7.15 (m, 5H, ArH), 3.65–3.45 (m, 2H, NCH2), 3.28–3.10 (m, 2H, NCH2), 1.58–1.27 (m, 6H, NCH2(C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2)3); 13C NMR (75 MHz, CDCl3) δ: 170.1, 136.3, 129.2, 128.2, 126.6, 48.5, 42.9, 26.3, 25.5, 24.4; MS (EI, 70 eV) m/z (% rel.): 189 [M+˙] (35), 188 (100), 105 (75), 77 (41). Anal. calcd for C12H15NO: C, 76.16; H, 7.99; N, 7.40. Found: C, 76.29; H, 8.01; N, 7.41.
2)3); 13C NMR (75 MHz, CDCl3) δ: 170.1, 136.3, 129.2, 128.2, 126.6, 48.5, 42.9, 26.3, 25.5, 24.4; MS (EI, 70 eV) m/z (% rel.): 189 [M+˙] (35), 188 (100), 105 (75), 77 (41). Anal. calcd for C12H15NO: C, 76.16; H, 7.99; N, 7.40. Found: C, 76.29; H, 8.01; N, 7.41.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v): Rf = 0.59; 1H NMR (300 MHz, CDCl3) δ: 7.60–6.90 (m, 10H, ArH), 4.90–4.42 (m, 2H, NCH2Ph), 3.78–3.05 (m, 2H, NCH2), 1.38–1.02 (m, 3H, CH2C
30 v/v): Rf = 0.59; 1H NMR (300 MHz, CDCl3) δ: 7.60–6.90 (m, 10H, ArH), 4.90–4.42 (m, 2H, NCH2Ph), 3.78–3.05 (m, 2H, NCH2), 1.38–1.02 (m, 3H, CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (75 MHz, CDCl3) δ:170.8, 133.4, 130.1, 129.8, 128.9, 128.5, 128.3, 127.6, 126.6, 53.9, 42.9, 13.9; MS (EI, 70 eV) m/z (% rel.): 239 [M+˙] (55), 238 (40), 210 (10), 148 (5), 134 (6), 105 (100), 91 (20), 77 (35). Anal. calcd for C16H17NO: C, 80.30; H, 7.16; N, 5.85. Found: C, 80.41; H, 7.17; N, 5.86.
3); 13C NMR (75 MHz, CDCl3) δ:170.8, 133.4, 130.1, 129.8, 128.9, 128.5, 128.3, 127.6, 126.6, 53.9, 42.9, 13.9; MS (EI, 70 eV) m/z (% rel.): 239 [M+˙] (55), 238 (40), 210 (10), 148 (5), 134 (6), 105 (100), 91 (20), 77 (35). Anal. calcd for C16H17NO: C, 80.30; H, 7.16; N, 5.85. Found: C, 80.41; H, 7.17; N, 5.86.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
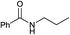
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v): Rf = 0.38, 1H NMR (300 MHz, CDCl3) δ: 7.95–7.70 (m, 2H, ArH), 7.51–7.28 (m, 3H, ArH), 3.36 (t, J = 7.2 Hz, 2H, NHC
30 v/v): Rf = 0.38, 1H NMR (300 MHz, CDCl3) δ: 7.95–7.70 (m, 2H, ArH), 7.51–7.28 (m, 3H, ArH), 3.36 (t, J = 7.2 Hz, 2H, NHC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2), 1.72–1.54 (m, 2H, NHCH2C
2), 1.72–1.54 (m, 2H, NHCH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2), 0.90 (t, J = 7.2, Hz, 3H, C
2), 0.90 (t, J = 7.2, Hz, 3H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3CH2); 13C NMR (75 MHz, CDCl3) δ: 168.9, 131.9, 129.9, 128.5, 127.4, 42.3, 22.5, 11.3; MS (EI, 70 eV) m/z (% rel.): 163 [M+˙] (40), 148 (5), 134 (10), 105 (100), 77 (40). Anal. calcd for C10H13NO: C, 73.59; H, 8.03; N, 8.58. Found: C, 73.69; H, 8.04; N, 8.59.
3CH2); 13C NMR (75 MHz, CDCl3) δ: 168.9, 131.9, 129.9, 128.5, 127.4, 42.3, 22.5, 11.3; MS (EI, 70 eV) m/z (% rel.): 163 [M+˙] (40), 148 (5), 134 (10), 105 (100), 77 (40). Anal. calcd for C10H13NO: C, 73.59; H, 8.03; N, 8.58. Found: C, 73.69; H, 8.04; N, 8.59.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v): Rf = 0.58, 1H NMR (300 MHz, CDCl3) δ: 8.35–7.05 (m, 11H, ArH and NH), 13C NMR (75 MHz, CDCl3) δ: 165.9, 137.9, 133.2, 130.1, 129.0, 128.7, 127.1, 124.3, 120.3 MS (EI, 70 eV) m/z (% rel.): 197 [M+˙] (60), 105 (100), 77 (50). Anal. calcd for C13H11NO: C, 79.16; H, 5.62; N, 7.10. Found: C, 79.27; H, 5.63; N, 7.11.
30 v/v): Rf = 0.58, 1H NMR (300 MHz, CDCl3) δ: 8.35–7.05 (m, 11H, ArH and NH), 13C NMR (75 MHz, CDCl3) δ: 165.9, 137.9, 133.2, 130.1, 129.0, 128.7, 127.1, 124.3, 120.3 MS (EI, 70 eV) m/z (% rel.): 197 [M+˙] (60), 105 (100), 77 (50). Anal. calcd for C13H11NO: C, 79.16; H, 5.62; N, 7.10. Found: C, 79.27; H, 5.63; N, 7.11.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v): Rf = 0.62; 1H NMR (300 MHz, CDCl3) δ: 8.22 (d, J = 9.0 Hz, 2H), 7.88 (d, J = 9.0 Hz, 2H, ArH), 7.25–7.15 (m, 3H, ArH), 7.14–7.04 (m, 2H, ArH), 6.24 (d, J = 8.1 Hz, 1H, NH), 4.48–4.32 (m, 1H, CHCO), 3.39–3.23 (m, 1H, C
10 v/v): Rf = 0.62; 1H NMR (300 MHz, CDCl3) δ: 8.22 (d, J = 9.0 Hz, 2H), 7.88 (d, J = 9.0 Hz, 2H, ArH), 7.25–7.15 (m, 3H, ArH), 7.14–7.04 (m, 2H, ArH), 6.24 (d, J = 8.1 Hz, 1H, NH), 4.48–4.32 (m, 1H, CHCO), 3.39–3.23 (m, 1H, C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 2CH3), 3.09–2.83 (m, 5H, CH2Ph and NCH2), 0.99–0.80 (m, 6H, CH2C
2CH3), 3.09–2.83 (m, 5H, CH2Ph and NCH2), 0.99–0.80 (m, 6H, CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (75 MHz, CDCl3) δ: 169.3, 149.8, 146.3, 135.4, 129.5, 128.6, 128.2, 127.3, 123.9, 54.5, 41.5, 40.7, 13.9, 12.5; MS (EI, 70 eV) m/z (% rel.): 405 [M+˙] (2), 314 (51), 305 (100), 203 (44), 100 (55), 72 (61). Anal. calcd for C19H23N3O5S: C, 56.28; H, 5.72; N, 10.36; S, 7.91. Found: C, 56.43; H, 5.74; N, 10.33; S, 7.88.
3); 13C NMR (75 MHz, CDCl3) δ: 169.3, 149.8, 146.3, 135.4, 129.5, 128.6, 128.2, 127.3, 123.9, 54.5, 41.5, 40.7, 13.9, 12.5; MS (EI, 70 eV) m/z (% rel.): 405 [M+˙] (2), 314 (51), 305 (100), 203 (44), 100 (55), 72 (61). Anal. calcd for C19H23N3O5S: C, 56.28; H, 5.72; N, 10.36; S, 7.91. Found: C, 56.43; H, 5.74; N, 10.33; S, 7.88.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v): Rf = 0.72; 1H NMR (300 MHz, CDCl3) δ: 8.32 (d, J = 8.1 Hz, 2H, ArH), 8.04 (d, J = 8.1 Hz, 2H, ArH), 6.24 (d, J = 8.7 Hz, 1H, NH), 4.32–4.18 (m, 1H, CHCO), 3.30–3.05 (m, 4H, NCH2), 1.33 (d, J = 6.9 Hz, 3H, CHC
10 v/v): Rf = 0.72; 1H NMR (300 MHz, CDCl3) δ: 8.32 (d, J = 8.1 Hz, 2H, ArH), 8.04 (d, J = 8.1 Hz, 2H, ArH), 6.24 (d, J = 8.7 Hz, 1H, NH), 4.32–4.18 (m, 1H, CHCO), 3.30–3.05 (m, 4H, NCH2), 1.33 (d, J = 6.9 Hz, 3H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.09 (t, J = Hz, 3H, CH2C
3), 1.09 (t, J = Hz, 3H, CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 0.92 (t, J = Hz, 3H, CH2C
3), 0.92 (t, J = Hz, 3H, CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (75 MHz, CDCl3) δ: 170.1, 149.9, 146.3, 128.4, 124.1, 49.2, 41.6, 40.6, 20.5, 14.3, 12.6; MS (EI, 70 eV) m/z (% rel.): 229 (69), 186 (15), 122 (10), 100 (95), 72 (100). Anal. calcd for C13H19N3O5S: C, 47.41; H, 5.81; N, 12.76; S, 9.74. Found: C, 47.55; H, 5.82; N, 12.80; S, 9.76.
3); 13C NMR (75 MHz, CDCl3) δ: 170.1, 149.9, 146.3, 128.4, 124.1, 49.2, 41.6, 40.6, 20.5, 14.3, 12.6; MS (EI, 70 eV) m/z (% rel.): 229 (69), 186 (15), 122 (10), 100 (95), 72 (100). Anal. calcd for C13H19N3O5S: C, 47.41; H, 5.81; N, 12.76; S, 9.74. Found: C, 47.55; H, 5.82; N, 12.80; S, 9.76.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v): Rf = 0.88; 1H NMR (300 MHz, CDCl3) δ: 7.91 (s, 1H, NH), 7.55 (d, J = 9.0 Hz, 2H, ArH), 7.01 (d, J = 9.0 Hz, 2H, ArH), 2.29 (s, 3H, CH3CO), 2.17 (s, 3H, CH3CO), 13C NMR (75 MHz, CDCl3) δ 169.8, 168.6, 147.0, 135.9, 121.8, 120.8, 24.4, 21.1; MS (EI, 70 eV) m/z (% rel.): 193 [M+˙] (10), 151 (55), 109 (100), 80 (10). Anal. calcd for C10H11NO3: C, 62.17; H, 5.74; N, 7.25. Found: C, 62.26; H, 5.75; N, 7.26.
30 v/v): Rf = 0.88; 1H NMR (300 MHz, CDCl3) δ: 7.91 (s, 1H, NH), 7.55 (d, J = 9.0 Hz, 2H, ArH), 7.01 (d, J = 9.0 Hz, 2H, ArH), 2.29 (s, 3H, CH3CO), 2.17 (s, 3H, CH3CO), 13C NMR (75 MHz, CDCl3) δ 169.8, 168.6, 147.0, 135.9, 121.8, 120.8, 24.4, 21.1; MS (EI, 70 eV) m/z (% rel.): 193 [M+˙] (10), 151 (55), 109 (100), 80 (10). Anal. calcd for C10H11NO3: C, 62.17; H, 5.74; N, 7.25. Found: C, 62.26; H, 5.75; N, 7.26.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v): Rf = 0.81; 1H NMR (300 MHz, CDCl3) δ: 7.26–7.10 (m, 5H, ArH), 5.39 (d, J = 9 Hz, 1H, NH), 4.74–4.64 (m, 1H, CHCO), 3.53–3.41 (m, 1H, C
30 v/v): Rf = 0.81; 1H NMR (300 MHz, CDCl3) δ: 7.26–7.10 (m, 5H, ArH), 5.39 (d, J = 9 Hz, 1H, NH), 4.74–4.64 (m, 1H, CHCO), 3.53–3.41 (m, 1H, C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 2CH3); 3.11–2.80 (m, 5H CH2Ph, C
2CH3); 3.11–2.80 (m, 5H CH2Ph, C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 2CH3), 1.37 (s, 9H, tBu), 0.99 (t, J = 7.2 Hz, 3H, C
2CH3), 1.37 (s, 9H, tBu), 0.99 (t, J = 7.2 Hz, 3H, C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 3CH2N), 0.92 (t, J = 7.2 Hz, 3H, C
3CH2N), 0.92 (t, J = 7.2 Hz, 3H, C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 3CH2N), 13C NMR (75 MHz, CDCl3) δ 170.8, 154.9, 136.5, 129.5, 128.3, 126.7, 79.5, 51.3, 41.5, 40.4, 28.2, 14.0; MS (EI, 70 eV) m/z (% rel.): 320 [M+˙] (2), 229 (6), 220 (20), 164 (45), 129 (40), 120 (100), 100 (35), 91 (20), 72 (40), 57 (70). Anal. calcd for C18H28N2O3: C, 67.47; H, 8.81; N, 8.74. Found: C, 67.51; H, 8.87; N, 8.75.
3CH2N), 13C NMR (75 MHz, CDCl3) δ 170.8, 154.9, 136.5, 129.5, 128.3, 126.7, 79.5, 51.3, 41.5, 40.4, 28.2, 14.0; MS (EI, 70 eV) m/z (% rel.): 320 [M+˙] (2), 229 (6), 220 (20), 164 (45), 129 (40), 120 (100), 100 (35), 91 (20), 72 (40), 57 (70). Anal. calcd for C18H28N2O3: C, 67.47; H, 8.81; N, 8.74. Found: C, 67.51; H, 8.87; N, 8.75.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v): Rf = 0.88; 1H NMR (300 MHz, CDCl3) δ: 8.17 (d, J = 8.7 Hz, 2H, ArH), 7.93 (d, J = 8.7 Hz, 2H, ArH), 7.34–7.23 (m, 5H, ArH), 5.94 (d, J = 8.2, 1H, NH), 5.55 (d, J = 8.1, 1H, NH), 4.97–4.86 (m, 1H, CHPh), 3.90–3.79 (m, 1H, CHCO), 1.44 (d, J = 6.9 Hz, 3H, C
30 v/v): Rf = 0.88; 1H NMR (300 MHz, CDCl3) δ: 8.17 (d, J = 8.7 Hz, 2H, ArH), 7.93 (d, J = 8.7 Hz, 2H, ArH), 7.34–7.23 (m, 5H, ArH), 5.94 (d, J = 8.2, 1H, NH), 5.55 (d, J = 8.1, 1H, NH), 4.97–4.86 (m, 1H, CHPh), 3.90–3.79 (m, 1H, CHCO), 1.44 (d, J = 6.9 Hz, 3H, C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 3CHPh), 1.39 (d, J = 6.9 Hz, 3H, C
3CHPh), 1.39 (d, J = 6.9 Hz, 3H, C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 3CHCO); 13C NMR (75 MHz, CDCl3) δ 168.1, 152.1, 142.1, 137.9, 128.8, 128.2, 127.8, 125.9, 124.3, 55.1, 49.7, 21.8, 20.1; anal. calcd for C17H19N3O5S: C, 54.10; H, 5.07; N, 11.13; S, 8.50. Found: C, 54.18; H, 5.15; N, 11.28; S, 8.61. ESI-QTOF-MS: 378.1063 (M + H)+, 400.0944 (M + Na)+.
3CHCO); 13C NMR (75 MHz, CDCl3) δ 168.1, 152.1, 142.1, 137.9, 128.8, 128.2, 127.8, 125.9, 124.3, 55.1, 49.7, 21.8, 20.1; anal. calcd for C17H19N3O5S: C, 54.10; H, 5.07; N, 11.13; S, 8.50. Found: C, 54.18; H, 5.15; N, 11.28; S, 8.61. ESI-QTOF-MS: 378.1063 (M + H)+, 400.0944 (M + Na)+.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v): Rf = 0.88; 1H NMR (300 MHz, CDCl3) δ: 8.35 (d, J = 9 Hz, 2H, ArH), 8.05 (d, J = 9 Hz, 2H, ArH), 7.38–7.21 (m, 5H, ArH), 5.97 (d, J = 7.2 Hz 1H, NH), 5.60 (sbroad, 1H, NH), 4.98–4.88 (m, 1H, CHPh), 3.91–3.82 (m, 1H, CHCO), 1.40 (d, J = 6.9 Hz, 3H, C
30 v/v): Rf = 0.88; 1H NMR (300 MHz, CDCl3) δ: 8.35 (d, J = 9 Hz, 2H, ArH), 8.05 (d, J = 9 Hz, 2H, ArH), 7.38–7.21 (m, 5H, ArH), 5.97 (d, J = 7.2 Hz 1H, NH), 5.60 (sbroad, 1H, NH), 4.98–4.88 (m, 1H, CHPh), 3.91–3.82 (m, 1H, CHCO), 1.40 (d, J = 6.9 Hz, 3H, C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 3CHPh), 1.35 (d, J = 6.9 Hz, 3H, C
3CHPh), 1.35 (d, J = 6.9 Hz, 3H, C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 3CHCO); 13C NMR (75 MHz, CDCl3) δ 168.1, 151.8, 141.2, 136.9, 128.8, 128.4, 127.8, 125.9, 124.3, 52.5, 49.1, 22.8, 21.1; anal. calcd for C17H19N3O5S: C, 54.10; H, 5.07; N, 11.13; S, 8.50. Found: C, 54.15; H, 5.09; N, 11.23; S, 8.59. ESI-QTOF-MS: 378.1092 (M + H)+, 400.0939 (M + Na)+.
3CHCO); 13C NMR (75 MHz, CDCl3) δ 168.1, 151.8, 141.2, 136.9, 128.8, 128.4, 127.8, 125.9, 124.3, 52.5, 49.1, 22.8, 21.1; anal. calcd for C17H19N3O5S: C, 54.10; H, 5.07; N, 11.13; S, 8.50. Found: C, 54.15; H, 5.09; N, 11.23; S, 8.59. ESI-QTOF-MS: 378.1092 (M + H)+, 400.0939 (M + Na)+.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
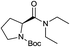
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v): Rf = 0.78; 1H NMR (500 MHz, CDCl3) two conformers (60*:40) δ: 4.55 (dd, J = 7.8 Hz, J = 2.9 Hz, 1H, CHCO), 4.41* (dd, J = 8.4, J = 3.8 Hz, 1H, CHCO), 3.63–3.15 (m, 6H, CH2C
30 v/v): Rf = 0.78; 1H NMR (500 MHz, CDCl3) two conformers (60*:40) δ: 4.55 (dd, J = 7.8 Hz, J = 2.9 Hz, 1H, CHCO), 4.41* (dd, J = 8.4, J = 3.8 Hz, 1H, CHCO), 3.63–3.15 (m, 6H, CH2C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 2NCO, C
2NCO, C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 2CH3), 2.21–1.91 (m, 2H, CH2C
2CH3), 2.21–1.91 (m, 2H, CH2C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 2CHCO), 1.87–1.71 (m, 2H, C
2CHCO), 1.87–1.71 (m, 2H, C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 2CH2CHCO), 1.41 and 1.47* (2 s, 9H, tBu), 1.24–1.15 (m, 3H, C
2CH2CHCO), 1.41 and 1.47* (2 s, 9H, tBu), 1.24–1.15 (m, 3H, C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 3CH2N), 1.13–1.03 (m, 3H, C
3CH2N), 1.13–1.03 (m, 3H, C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 3CH2N); 13C NMR (126 MHz, CDCl3) δ 172.06, 154.62, 154.07*, 79.63*, 79.33, 56.57*, 56.32, 47.08, 46.94*, 41.84, 41.05*, 40.84, 31.35*, 30.37, 28.62, 28.57*, 24.29, 23.53*, 14.85, 13.26*, 13.04; MS (EI, 70 eV) m/z (% rel.): 270 [M+˙] (1), 197 (18), 170 (37), 114 (88), 100 (29), 70 (100), 57 (43). Anal. calcd for C14H26N2O3: C, 62.19; H, 9.69; N, 10.36. Found: C, 62.25; H, 9.65; N, 10.30.
3CH2N); 13C NMR (126 MHz, CDCl3) δ 172.06, 154.62, 154.07*, 79.63*, 79.33, 56.57*, 56.32, 47.08, 46.94*, 41.84, 41.05*, 40.84, 31.35*, 30.37, 28.62, 28.57*, 24.29, 23.53*, 14.85, 13.26*, 13.04; MS (EI, 70 eV) m/z (% rel.): 270 [M+˙] (1), 197 (18), 170 (37), 114 (88), 100 (29), 70 (100), 57 (43). Anal. calcd for C14H26N2O3: C, 62.19; H, 9.69; N, 10.36. Found: C, 62.25; H, 9.65; N, 10.30.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v): Rf = 0.78; 1H NMR (500 MHz, CDCl3) two conformers (59*:41) δ: 4.53 (dd, J = 7.8 Hz, J = 3.0 Hz, 1H, CHCO), 4.38* (dd, J = 8.4 Hz, J = 3.8 Hz, 1H, CHCO), 3.60–3.11 (m, 6H, CH2C
30 v/v): Rf = 0.78; 1H NMR (500 MHz, CDCl3) two conformers (59*:41) δ: 4.53 (dd, J = 7.8 Hz, J = 3.0 Hz, 1H, CHCO), 4.38* (dd, J = 8.4 Hz, J = 3.8 Hz, 1H, CHCO), 3.60–3.11 (m, 6H, CH2C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 2NCO, C
2NCO, C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 2CH3), 2.19–1.90 (m, 2H, C
2CH3), 2.19–1.90 (m, 2H, C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 2CH2CHCO), 1.86–1.68 (m, 2H, C
2CH2CHCO), 1.86–1.68 (m, 2H, C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 2CH2CHCO), 1.38 and 1.34* (2s, 9H, tBu), 1.22–1.13 (m, 3H, C
2CH2CHCO), 1.38 and 1.34* (2s, 9H, tBu), 1.22–1.13 (m, 3H, C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 3CH2N), 1.10–1.01 (m, 3H, C
3CH2N), 1.10–1.01 (m, 3H, C![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) 3CH2N); 13C NMR (126 MHz, CDCl3) δ 172.10, 154.60, 154.03*, 79.53*, 79.28, 56.62*, 56.35, 46.95, 41.78, 40.96, 31.34*, 30.31, 28.58, 24.27, 23.44*, 14.76, 13.14; MS (EI, 70 eV) m/z (% rel.): 270 [M+˙] (1), 197 (19), 170 (40), 114 (94), 100 (30), 70 (100), 57 (45). Anal. calcd for C14H26N2O3: C, 62.19; H, 9.69; N, 10.36. Found: C, 62.23; H, 9.63; N, 10.31.
3CH2N); 13C NMR (126 MHz, CDCl3) δ 172.10, 154.60, 154.03*, 79.53*, 79.28, 56.62*, 56.35, 46.95, 41.78, 40.96, 31.34*, 30.31, 28.58, 24.27, 23.44*, 14.76, 13.14; MS (EI, 70 eV) m/z (% rel.): 270 [M+˙] (1), 197 (19), 170 (40), 114 (94), 100 (30), 70 (100), 57 (45). Anal. calcd for C14H26N2O3: C, 62.19; H, 9.69; N, 10.36. Found: C, 62.23; H, 9.63; N, 10.31.Synthesis of dipeptide methyl 3-methyl-2-(S)-(2-(R)-(4-nitrophenylsulfonamido)-propanamido)butanoate (20)
1 mmol of 2-(S)-(4-nitrophenylsulfonamido)propanoic acid is added to 1 mmol of (S)-methyl 2-amino-3-methylbutanoate hydrochloride and 5 mmol of triethylamine (Et3N) in dichloromethane, then 1 mmol of SOCl2 is added at room temperature. The mixture is stirred for 40 minutes at room temperature. The recovery of the reaction product is performed by evaporating the solvent under reduced pressure. The resulting residue is taken up in dichloromethane and washed first with 1 N HCl and then with 1 N NaOH. The organic phase was dried (Na2SO4), and evaporated to dryness to afford the corresponding dipeptide 20.White solid, 65%; mp 144–148 °C; TLC (eluent: chloroform/methanol 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v): Rf = 0.53; 1H NMR (500 MHz, CDCl3) δ: δ 8.27 (d, J = 8.8 Hz, 2H, ArH), 8.00 (d, J = 8.8 Hz, 2H, ArH), 6.47 (d, J = 8.7 Hz, 1H, N
10 v/v): Rf = 0.53; 1H NMR (500 MHz, CDCl3) δ: δ 8.27 (d, J = 8.8 Hz, 2H, ArH), 8.00 (d, J = 8.8 Hz, 2H, ArH), 6.47 (d, J = 8.7 Hz, 1H, N![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) SO2ArH), 5.93 (d, J = 8.5 Hz, 1H,N
SO2ArH), 5.93 (d, J = 8.5 Hz, 1H,N![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) CHCOOCH3), 4.31 (dd, J = 8.5 Hz, J = 4.8 Hz, 1H, CHCOOCH3), 4.02–3.91 (m, 1H, NHC
CHCOOCH3), 4.31 (dd, J = 8.5 Hz, J = 4.8 Hz, 1H, CHCOOCH3), 4.02–3.91 (m, 1H, NHC![[H with combining overline]](https://www.rsc.org/images/entities/char_0048_0305.gif) (CH3)CO, 3.69 (s, 3H, OCH3), 2.12–2.04 (m, 1H, CH(CH3)2), 1.33 (d, J = 7.1 Hz, 3H, CH3CH), 0.84 (d, J = 6.9 Hz, 3H, CH(CH3)2), 0.80 (t, J = 6.9 Hz, 3H, CH(CH3)2). 13C NMR (126 MHz, CDCl3) δ 172.31, 171.13, 150.35, 145.75, 128.68, 124.60, 57.22, 52.88, 52.61, 31.47, 20.40, 19.07, 17.70; MS (EI, 70 eV) m/z (% rel.): 328 [M+˙ − COOMe] (28), 274 (8), 229 (100), 186 (27), 130 (27), 122 (21), 72 (14). Anal. calcd for C15H21N3O7S: C, 46.50; H, 5.46; N, 10.85; S, 8.28. Found: C, 46.38; H, 5.48; N, 10.90; S, 8.26.
(CH3)CO, 3.69 (s, 3H, OCH3), 2.12–2.04 (m, 1H, CH(CH3)2), 1.33 (d, J = 7.1 Hz, 3H, CH3CH), 0.84 (d, J = 6.9 Hz, 3H, CH(CH3)2), 0.80 (t, J = 6.9 Hz, 3H, CH(CH3)2). 13C NMR (126 MHz, CDCl3) δ 172.31, 171.13, 150.35, 145.75, 128.68, 124.60, 57.22, 52.88, 52.61, 31.47, 20.40, 19.07, 17.70; MS (EI, 70 eV) m/z (% rel.): 328 [M+˙ − COOMe] (28), 274 (8), 229 (100), 186 (27), 130 (27), 122 (21), 72 (14). Anal. calcd for C15H21N3O7S: C, 46.50; H, 5.46; N, 10.85; S, 8.28. Found: C, 46.38; H, 5.48; N, 10.90; S, 8.26.
Results and discussion
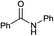
In order to verify the one-pot formation of the amide by using thionyl chloride as activating agent, we designed an experiment in which benzoic acid, the carboxylic acid chosen as model system, is treated, in the presence of triethylamine, with thionyl chloride and the amine (Scheme 1).1 mmol of benzoic acid was added to 1 mmol of diethylamine (Et2NH) and 3 mmol of triethylamine (Et3N) in dichloromethane, then 1 mmol of SOCl2 was added.
After 5 minutes stirring at room temperature, TLC analysis of the reaction mixture showed the complete conversion of benzoic acid.
The recovery of the reaction product was performed by evaporating the solvent under reduced pressure to remove traces of unreacted thionyl chloride. The resulting residue, after work up, provided the corresponding N,N-diethylbenzamide (1) in 86% overall yield (Table 2).
The molecular structure of 1 was assigned by 1H and 13C NMR spectroscopy and GC/MS analysis.
An additional experiment was performed to investigate the reaction progress in absence of the tertiary amine Et3N. To this aim 1 mmol of SOCl2 was added to 1 mmol of benzoic acid and 1 mmol of diethylamine (Et2NH) in dichloromethane at room temperature. After 20 minutes the reaction was not yet complete and a mixture of benzoic acid (65% yield) and N,N-diethylbenzamide (31% yield) was recovered.
The use of stoichiometric amounts of diethylamine, in absence of Et3N, leads to a lower conversion to amides since the diethylamine works also as a base to neutralize the hydrochloric acid that is generated during the reaction. This result demonstrated that the presence of the tertiary amine is essential to obtain the amide in high yields.
We also experienced that the outcome of the reaction is strongly dependent on the order of reagent addition. In fact, if benzoic acid is preliminarily added to thionyl chloride and Et2NH and Et3N are added subsequently, the reaction yield is lowered and after 5 minutes, the reaction is not yet complete. The obtained reaction product contained also the benzoyl chloride with a percentage of 35% as detected by GC/MS analysis.
Furthermore, it was verified that SOCl2 does not react with the amine. In fact, the N-benzyl-N-ethylamine was recovered unchanged after it was treated with SOCl2 at room temperature for 1 hour.
With the aim of investigating the reaction mechanism, we designed 13C NMR experiments by performing and monitoring the reactions directly into the NMR tube using CDCl3 as solvent and observing the chemical shifts of the carbonyl groups of the products present in the reaction mixture.
The purpose of these experiments was to identify the reactive intermediate generated during the one pot amide formation reaction. Therefore, we recorded preliminarily the 13C NMR spectra of benzoic acid and benzoyl chloride that exhibit carbonyl resonance signals respectively at 172.51 ppm and 168.38 ppm.
Subsequently in a NMR tube, benzoic acid (1 mmol) was treated with thionyl chloride (1 mmol) at room temperature in CDCl3 as solvent. The progress of the reaction was followed by recording the 13C NMR spectrum of the reaction mixture. After more than 2 hours 13C NMR spectrum showed the presence of the signal at (172.44 ppm) corresponding to the carbonyl group of benzoic acid and a signal of very low intensity, that can be just appreciated, relating to acyl chloride carbonyl group (168.52 ppm) This demonstrated that benzoyl chloride is formed hardly under these reaction conditions.
In an additional experiment, the same reaction was performed at room temperature by adding thionyl chloride (1 mmol) to a mixture of benzoic acid (1 mmol) and triethylamine (3 mmol) dissolved in CDCl3. In this case the reaction proceeds rapidly and in the 13C NMR spectrum it was observed immediately the disappearance of the signal relating to the resonance of benzoic acid carbonyl group and the appearance at 168.13 ppm of the signal generated by benzoyl chloride carbonyl group.
Finally, we reproduced, the one pot formation of N,N-diethylbenzamide (1) in a NMR tube, under the described conditions for the synthesis of amides 1–17, and immediately recorded the 13C NMR spectrum. In the obtained 13C NMR spectrum at once the presence of two resonances attributable to the carbonyl groups of N,N-diethylbenzamide at 171.36 ppm and of benzoyl chloride at 168.32 ppm was observed. After about 10 minutes, the re-recorded 13C NMR spectrum of the reaction mixture revealed only the presence of the resonance corresponding to the amidic carbonyl group.
These results could be justified by the action of tertiary amine that causes the formation of the carboxylate, which reacts quickly at room temperature with thionyl chloride to form the acid chloride that immediately reacts with the N,N-diethylamine to give the corresponding amide.
This experimental evidence suggests that benzoyl chloride is the reactive intermediate of the adopted one pot procedure (Scheme 2).
The reaction was subsequently extended to other aromatic substrates, in all experimented cases the reaction is complete within 5 minutes and has provided excellent yields (86–91%) (Table 2, 2–4).
The methodology was then applied to two long chain aliphatic carboxylic acids, palmitic and myristic acid. Also with these systems no changes were observed during the reaction, in fact the amide formation occurs even in short times and high yields (88–90%) (Table 2, 5, 6).
In order to evaluate the possible effects due to the steric hindrance of the amine, N-ethyl-N-isopropylamine, piperidine and N-benzyl-N-ethylamine were treated with benzoic acid under the described reaction conditions.
Significant effects due to steric hindrance of the amines were not detected. Both the reaction kinetics that the reaction yields are similar to those of the reaction with diethylamine (Table 2, 7–9).
Then, with the purpose to investigate the steric hindrance present on the carboxylic acid, the developed procedure was applied to two N-protected α-amino acids, N-nosyl-L-phenylalanine and N-nosyl-L-alanine.
N-Nosyl-L-phenylalanine and N-nosyl-L-alanine were converted into the corresponding N,N-diethylamides (Table 2, 12, 13) in 20 minutes and with yields slightly lower.
Based on these results it can be argued that steric hindrance offered by the groups on the nitrogen atom of the amine does not affect the reaction progress while the reaction times are longer and the reaction yields are a little lower when sterically hindered carboxylic acids are used.
The adopted procedure for obtaining N,N-diethylamides was also applied successfully to N-Boc-L-phenylalanine, a substrate bearing the acid labile group tert-butoxycarbonyl (Boc). The corresponding N,N-diethylamide (15) was obtained in 78% yield keeping unchanged the acid-sensitive Boc group (Table 2). On the contrary the application of the two-step amide synthesis, through the formation of acyl chloride, to N-Boc-L-phenylalanine does not work well due to the instability of the corresponding acyl chloride that, when isolated in the absence of the nucleophile, decomposes readily.
The described protocol was also employed to obtain secondary amides by using primary amines as nucleophilic reagents. To this aim, N-propylamine and aniline were selected as nucleophiles. The reactions afforded the corresponding amides in short times and high yields (Table 2, 10, 11) by demonstrating that the adopted one-pot procedure is also applicable successfully for obtaining secondary amides.
We also investigated the stereochemical aspects of the reaction by extending the developed procedure to the formation of a couple of diastereoisomeric amides. To this aim N-((R)-1-phenylethyl)-2-(S)-(4-nitrophenylsulfonamido)-propanamide (16) and N-((S)-1-phenylethyl)-2-(S)-(4-nitrophenylsulfonamido)propanamide (17) were synthesized by treating N-(4-nitrobenzenesulfonyl)-L-alanine with (R)-1-phenylethylamine and (S)-1-phenylethylamine respectively according the adopted conditions. The corresponding amides 16 and 17 were obtained in 68–75% yields after 20 minutes (Table 2). The 1H NMR and 13C NMR spectroscopic data of crude amides 16 and 17 did not show any signals from possible epimers resulting from an inversion of the configuration at the α-carbon atom of the alanine.
The absence of epimerization was also monitored by HPLC analysis of the crude amides 16 and 17 and of a suitably prepared mixture of the two diastereoisomers 16 and 17 (approx. 30%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70%). The two diastereoisomers were readily resolved by HPLC: the presence of two chromatographic peaks with retention times of 15.558 and 16.867 minutes was observed in the HPLC analysis of the mixture (Fig. 1B). Chromatograms of the single amide 16 (Fig. 1A) and 17 (Fig. 1C) showed the presence of a unique peak with retention times of 16.908 and 15.433 minutes respectively.
70%). The two diastereoisomers were readily resolved by HPLC: the presence of two chromatographic peaks with retention times of 15.558 and 16.867 minutes was observed in the HPLC analysis of the mixture (Fig. 1B). Chromatograms of the single amide 16 (Fig. 1A) and 17 (Fig. 1C) showed the presence of a unique peak with retention times of 16.908 and 15.433 minutes respectively.
HPLC analysis in combination with NMR data of the two diastereomeric amides 16 and 17, demonstrated the absence of epimerization products excluding any detectable epimerization throughout the synthetic process.
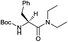
Furthermore, in order to evaluate the enantiomeric purity of N,N-diethylamides of N-Boc protected α-amino acids, two N,N-diethylamides of N-Boc-L-proline (18) and its enantiomer N-Boc-D-proline (19) were synthesized using the developed protocol (Table 2) and characterized by chiral GC/MS analysis.
The separation of L-amide 18 and D-amide 19 was achieved by chiral GC/MS analysis of a mixture of the two enantiomers 18 and 19 (approx. 70%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30%): the corresponding chromatogram showed the presence of two peaks with retention times of 114.08 and 114.73 minutes related to 18 and 19 respectively.
30%): the corresponding chromatogram showed the presence of two peaks with retention times of 114.08 and 114.73 minutes related to 18 and 19 respectively.
The chiral GC/MS analysis of the single amide 18 showed a unique peak at 113.97 min. While that of the single amide 19 showed two peaks at 113.88 and 114.75 minutes which corresponded to identical mass spectra. The estimated enantiomeric excess of D-amide 19 was 99.2%. The resulting ee was really satisfactory.
For completeness, since the amidation is mainly used in peptide synthesis, the developed protocol was also experimented for the synthesis of a small peptide. For this purpose the dipeptide system methyl 3-methyl-2-(S)-(2-(R)-(4-nitrophenylsulfonamido)-propanamido)butanoate (20) was prepared by treating N-4-nitrophenylsulfonyl-D-alanine (1 mmol) with L-valine methyl ester hydrochloride (1 mmol) under the adopted reaction conditions using 5 equivalents of Et3N. The methodology was successful in giving, after 40 minutes, the desired dipeptide derivative 20, which was isolated in good yields (65%, Table 2).
A last experiment was performed to verify the preparation of the amides in large scale. 10 grams of benzoic acid were treated with Et2NH (8.5 mL) and Et3N (34.5 mL) in dichloromethane, then SOCl2 (8.5 mL) was added to the reaction mixture at room temperature. The reaction, monitored by TLC (Et2O/petroleum ether, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v), was complete after 15 minutes and provided the N,N-diethylbenzamide (1) in 78% yield. This experiment showed that our protocol can be also applied successfully to synthesis of amides on a multi-gram scale.
3, v/v), was complete after 15 minutes and provided the N,N-diethylbenzamide (1) in 78% yield. This experiment showed that our protocol can be also applied successfully to synthesis of amides on a multi-gram scale.
Finally, we evaluated the chemoselectivity of the reaction by using a nucleophile containing both an amino and a phenolic function. According to the developed procedure, p-amino phenol was treated with an equimolar amount of acetic acid and thionyl chloride. After 15 minutes, TLC analysis (Et2O/petroleum ether, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) showed the complete conversion of the starting material. The reaction workup afforded p-acetamidophenyl acetate in 48% yield (Table 2, 14). Therefore, the acylation reaction takes place both on the amino and phenolic function without any chemoselectivity.
3, v/v) showed the complete conversion of the starting material. The reaction workup afforded p-acetamidophenyl acetate in 48% yield (Table 2, 14). Therefore, the acylation reaction takes place both on the amino and phenolic function without any chemoselectivity.
Conclusions
Thionyl chloride works as coupling reagent and allows obtaining, at room temperature and in short times, secondary and tertiary amides in high yields without need of further purification. Furthermore, the adopted conditions did not cause any significant loss of the optical integrity at the chiral centres of the precursors as confirmed by HPLC and NMR analysis of a couple of synthesized diastereoisomeric amides and by the high enantiomeric excess estimated for N,N-diethylamides of N-Boc-D-proline.The reaction progress is affected by steric hindrance only when bulky groups are present on the carboxylic acid molecule. The synthesis of amides is performed in an efficient one-pot procedure under mild reaction conditions; the order of reagent addition is predetermined for obtaining the amides in high yields.
The reaction mechanism was investigated by performing 13C NMR experiments, which showed, as the reactive intermediate is the acyl chloride. This is formed easily from the carboxylate generated in situ from the corresponding carboxylic acid in the presence of the tertiary amine.
Our approach was successfully extended to α-amino acids bearing acid-sensitive groups and to the synthesis of a dipeptide system; these results prove the applicative validity of the developed procedure also in the peptide synthesis.
Finally, the reaction can be also applied for large-scale synthesis of amides demonstrating its applicability for amide syntheses of preparative and semi-industrial interest.
Acknowledgements
This work was supported by grants from MIUR (Ministero Italiano dell'Università e della Ricerca, ex-60% funds).References
- (a) L. Stryer, Biochemistry, ed. W. H. Freeman, New York, 4th edn, 1995, p. 17 Search PubMed; (b) V. A. K. Ghose, N. Viswanadhan and J. J. Wendoloski, Comb. Chem., 1999, 1, 55 CrossRef; (c) E. Valeur and M. Bradley, Chem. Soc. Rev., 2009, 38, 606–631 RSC; (d) V. R. Pattabiraman and J. W. Bode, Nature, 2011, 480, 471–479 CrossRef CAS PubMed; (e) J. K. Park, W. K. Shin and D. K. An, Bull. Korean Chem. Soc., 2013, 34, 1592–1594 CrossRef CAS; (f) X. Yu and D. Sun, Molecules, 2013, 18, 6230–6268 CrossRef CAS PubMed; (g) J. D. Goodreid, P. A. Duspara, C. Bosch and R. A. Batey, J. Org. Chem., 2014, 79, 943–954 CrossRef CAS PubMed.
- (a) L. Zhang, X. Wang, J. Wang, N. Grinberg, D. K. Krishnamurthy and C. H. Senanayake, Tetrahedron Lett., 2009, 50, 2964–2966 CrossRef CAS; (b) R. M. Lanigan and T. D. Sheppard, Eur. J. Org. Chem., 2013, 7453–7465 CrossRef CAS; (c) K. B. Zambro, S. R. Dubbaka, D. Markovic, E. Moreno-Clavijo and P. Vogel, Org. Lett., 2013, 15, 2550–2553 CrossRef PubMed; (d) K. Nakamura, T. Nakajima, T. Aoyama, S. Okitsu and M. Koyama, Tetrahedron, 2014, 70, 8097–8107 CrossRef CAS.
- G. Johansson, Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, New York, 2004, vol. 2, pp. 442–463 Search PubMed.
- (a) L. Amarnath, I. Andrews, R. Bandichhor, A. Bhattacharya, P. Dunn, J. Hayler, W. Hinkley, N. Holub, D. Hughes, L. Humphreys, B. Kaptein, H. Krishnen, K. Lorenz, S. Mathew, G. Nagaraju, T. Rammeloo, P. Richardson, L. Wang, A. Wells and T. White, Org. Process Res. Dev., 2012, 16, 535–544 CrossRef; (b) R. Bandichhor, A. Bhattacharya, L. Diorazio, P. Dunn, K. Fraunhoffer, F. Gallou, J. Hayler, M. Hickey, W. Hinkley, D. Hughes, L. Humphreys, B. Kaptein, S. Mathew, T. Rammeloo, P. Richardson and T. White, Org. Process Res. Dev., 2013, 17, 615–626 CrossRef CAS.
- D. J. C. Constable, P. J. Dunn, J. D. Hayler, G. R. Humphrey, J. L. Leazer Jr, R. J. Linderman, K. Lorenz, J. Manley, B. A. Pearlman, A. Wells, A. Zaks and T. Y. Zhang, Green Chem., 2007, 9, 411–420 RSC.
- C. A. G. N. Montalbetti and V. Falque, Tetrahedron, 2005, 61, 10827–10852 CrossRef CAS.
- I. Weisz, J. Roboz and G. Bekesi, Tetrahedron Lett., 1996, 37, 563–566 CrossRef CAS.
- L. A. Rozov, P. W. Rafalko, S. M. Evans, L. Brockunier and K. J. Ramig, J. Org. Chem., 1995, 60, 1319–1325 CrossRef CAS.
- L. A. Carpino, B. J. Cohen Jr, K. E. Stephens, Y. Sadat-Aalaee, J.-H. Tien and D. C. Langridge, J. Org. Chem., 1986, 51, 3732 CrossRef CAS.
- A. Wissner and C. V. J. Grudzinskas, J. Org. Chem., 1978, 43, 3972–3974 CrossRef CAS.
- G. Prabhu, H. Basavaprabhu, N. Narendra, T. M. Vishwanatha and V. V. Sureshbabu, Tetrahedron, 2015, 71, 2785–2832 CrossRef CAS.
- G. Blotny, Tetrahedron, 2006, 62, 9507–9522 CrossRef CAS.
- C. E. Garrett, X. Jiang, K. Prasad and O. Repič, Tetrahedron Lett., 2002, 43, 4161–4165 CrossRef CAS.
- A. Devos, J. Remion, A.-M. Frisque-Hesbain, A. Colens and L. J. Ghosez, J. Chem. Soc., Chem. Commun., 1979, 1180–1181 RSC.
- E. Falb, T. Yechezkel, Y. Salitra and C. Gilon, J. Pept. Res., 1999, 53, 507–517 CrossRef CAS PubMed.
- (a) J. P. E. Human and J. A. Mills, Nature, 1946, 158, 877 CrossRef CAS PubMed; (b) F. Matsuda, M. Yanaqiya and T. Matsumoto, Tetrahedron Lett., 1982, 23, 4043–4046 CrossRef CAS.
- F. Matsuda, S. Itoh, N. Hattori, M. Yanagiya and T. Matsumoto, Tetrahedron, 1985, 41, 3625–3631 CrossRef CAS.
- R. J. Cvetovich and L. DiMichele, Org. Process Res. Dev., 2006, 10, 944–946 CrossRef CAS.
- J. Y. L. Chung, R. J. Cvetovich, M. McLaughlin, J. Amato, F.-R. Tsay, M. Jensen, S. Weissman and D. Zewge, J. Org. Chem., 2006, 71, 8602–8609 CrossRef CAS PubMed.
- O. Jacobson, Y. Bechor, A. Icar, N. Novak, A. Birman, H. Marom, L. Fadeeva, E. Golan, I. Leibovitch, M. Gutman, E. Even-Sapir, R. Chisin, M. Gozin and E. Mishani, Bioorg. Med. Chem., 2005, 13, 6195–6205 CrossRef CAS PubMed.
- J. J. Morris, L. R. Hughes, A. T. Glen and P. J. Taylor, J. Med. Chem., 1991, 34, 447–455 CrossRef CAS PubMed.
- Y. Ito, I. Washio and M. Ueda, Macromolecules, 2008, 41, 2778–3278 CrossRef CAS.
- S. Tortoioli, D. Marchal, M. Kesselgruber, T. Pabst, W. Skranc and S. Abele, Org. Process Res. Dev., 2014, 18, 1759–1762 CrossRef CAS.
- T. Kumagai, T. Anki, T. Ebi, A. Konishi, K. Matsumoto, H. Kurata, T. Kubo, K. Katsumoto, C. Kitamura and T. Kawase, Tetrahedron, 2010, 66, 8968–8973 CrossRef CAS.
- S. Pass, B. Amit and A. Patchornik, J. Am. Chem. Soc., 1981, 103, 7675–7676 CrossRef.
- (a) M. M. Joullié and K. M. Lassen, ARKIVOC, 2010, 189–250 Search PubMed; (b) C. Najera, Synlett, 2002, 1388–1403 CrossRef CAS.
- (a) M. L. Di Gioia, A. Barattucci, P. Bonaccorsi, A. Leggio, L. Minuti, E. Romio, A. Temperini and C. Siciliano, RSC Adv., 2014, 4, 2678–2686 RSC; (b) O. Marder and F. Albericio, Chim. Oggi, 2003, 21, 35–40 CAS.
- R. De Marco, M. L. Di Gioia, A. Leggio, A. Liguori, F. Perri, C. Siciliano and M. C. Viscomi, Amino Acids, 2010, 38, 691–700 CrossRef PubMed.
- A. Leggio, M. L. Di Gioia, F. Perri and A. Liguori, Tetrahedron, 2007, 63, 8164–8173 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra for compounds 1–20. 13C NMR spectra for elucidation of reaction mechanism, HPLC analyses of compounds 16, 17 and of a mixture of 16 and 17. Chiral GC/MS analyses of amides 18, 19 and a mixture of 18 and 19. See DOI: 10.1039/c5ra24527c |
| This journal is © The Royal Society of Chemistry 2016 |