Conversion of SPORL pretreated Douglas fir forest residues into microbial lipids with oleaginous yeasts†‡
Abstract
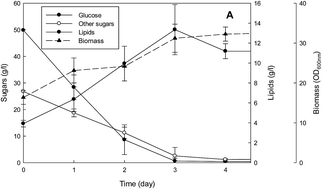
Douglas fir is the dominant commercial tree grown in the United States. In this study Douglas fir residue was converted to single cell oils (SCO) using oleaginous yeasts. Monosaccharides were extracted from the woody biomass by pretreating with sulfite and dilute sulfuric acid (SPORL process) and hydrolyzing using commercial cellulases. A new SPORL process that uses pH profiling was compared to the traditional method. Both processes yielded 77 g l−1 concentration of sugars. The SPORL generated sugars were evaluated for conversion to SCO using yeasts Lipomyces tetrasporus and Yarrowia lipolytica in batch cultures containing SPORL sugars diluted to 60% v/v supplemented with nitrogen at an appropriate C : N ratio of 75 : 1. An extended lag phase was observed for both yeasts, which was eliminated by including SPORL sugars diluted to 40% v/v in the seed cultures for acclimation. The maximum lipid concentrations were 3.18–5.13 g l−1. This corresponded to yields of 0.06–0.17 g lipid per g beginning sugars and productivities of 0.99–1.42 g l−1 d−1. Lipid concentrations for L. tetrasporus were further amplified by using two schemes incorporating multiple batch cultures. In the first, the yeast was grown in 40% v/v SPORL sugars and the entire contents of this fermentation transferred to undiluted SPORL sugars not supplemented with nitrogen. The result was the production of 13.4 g l−1 lipids within 3 days. This corresponds to a yield of 0.174 g g−1 and a productivity of 4.47 g l−1 d−1. The second approach was to thrice transfer the yeast cells in 60% v/v SPORL sugars supplemented with limited nitrogen to promote further lipid formation. The end result was 18.1 g l−1 of lipids with a process yield and productivity of 0.104 g g−1 and 1.29 g l−1 d−1, respectively. This is the first report that the authors are aware of demonstrating the feasibility of converting unconditioned woody biomass to single cell oil.

 Please wait while we load your content...
Please wait while we load your content...