Array of bis-quaternary ammonium surfactant tailored Cu(2−x)Te quantum dots with amended functional assets†
Deepika Jamwala,
Dolly Ranaa,
Dinesh Pathak*b,
Pankaj Raizadaa and
Pankaj Thakur *ac
*ac
aSchool of Chemistry, Faculty of Basic Sciences, Shoolini University, Solan, HP 173212, India. E-mail: Pankaj.thakur@iit.it; chempank@gmail.com
bFaculty of Chemical Technology, University of Pardubice, Pardubice 53210, Czech Republic. E-mail: dineshpathak80@gmail.com
cIstituto Italiano Di Tecnologia (Center for Advanced Biomaterials for Healthcare), Naples, 80125, Italy
First published on 25th January 2016
Abstract
Building on previous work, many studies have focussed on metal chalcogenide nanomaterials for diverse applications such as photothermal ablation of tumor cells and photothermal therapy, etc. Despite the large amount of related work, the available literature suggests that the size-dependent morphological impacts and similar characteristics have not been proposed for the aforementioned variety of chalcogenides. Explicitly, we present a simple synthetic pathway for the synthesis of copper telluride [Cu(2−x)Te] nanoparticles (NPs) stabilized by highly hydrophobic surfactants. The structural, optical and electrical properties were examined in relation to the cumulative diameter of the copper telluride NPs using various surfactants. Transmission electron micrography (TEM) suggests the increasing hydrophobic character of the surfactants is a factor for the morphological changes in the copper telluride NPs. Electrical conductivity studies of copper telluride nanoparticles were carried out at room temperature. The current–voltage (I–V) curves are linearly symmetric towards the origin inferring that the contacts are ohmic and the resistance of the samples is in the range from 1–5 Ω. UV-vis spectroscopy gave optical band gap values of 3.42 eV, 3.40 eV and 3.37 eV for the (12-2-12), (14-2-14) and (16-2-16) capped copper telluride NPs, respectively. A change in the band gap with diameter demonstrates a blue shift, which may be attributed to a quantum size effect in the NPs. Broadening of the photoluminescence (PL) peak may suggest strain amongst the nanoparticles instigated by size induced quantization effects.
1. Introduction
Semiconductor nanocrystals and more precisely metal chalcogenides have been habitually commended extensively for their amazing optical and electronic properties and photothermal and thermal ablation applications. Transition-metal chalcogenides in nanocrystalline form exhibit various interesting and useful characteristic properties different to their bulk equivalents.1,2 Quantum dots have attracted noticeable attention due to their novel electronic and optical properties, which can be tuned by the quantum size effect.3 Quantum dots perform essentially as a three-dimensional potential well for electrons. They have already been used successfully to improve the performance of devices such as light-emitting diodes,4 lasers5 and photodetectors,6 etc. These are attractive materials due to their excellent properties for third generation devices and these magnificent properties can be tuned by changing the size, morphology and composition of the nanoparticles.7As a repercussion of the quantum confinement effect, binary chalcogenide semiconductors have earned attention due to their size-dependent optical and physical properties.8–10 Our inspiration for the present work grew from our recent work, whereby metal chalcogenides were studied with a single surfactant molecule. Moreover, we didn’t encounter much research devoted to the hydrophobic character of three different stabilizing molecules on copper telluride nanoparticles. Copper telluride (group I–VI compounds) has been considered as a promising semiconductor with a direct band gap between 1.1 and 1.5 eV having wide applications such as solar cells,11,12 super ionic materials,13 optical recording material,14 optical filters,15 thermoelectric cooling materials,16 etc. In the present work, we report an array of copper telluride quantum dots with superior and different eV values, stabilized with assorted bis-quaternary ammonium surfactants resulting in well-definedNPs.17 Copper telluride (CuxTe) has different crystal structures depending upon the value of x (1 < x < 2) with diverse morphologies. Most prominent among them are spheres,18 rods,19 plates,20 wires,21 films,22 sheets,23 and cubic structures.24 Until now, a variety of synthetic strategies have been developed to design copper telluride semiconductor nanocrystals including solvothermal synthesis,25 sonochemical synthesis,26 elemental directed aqueous solution synthesis,27 microwave-assisted,28 ultrasonic irradiation,29 vacuum deposition,30 hydrothermal synthesis,31 etc. Recently, Zhang et al. reported various nanostructures of copper telluride (hexagonal plates, rods, sheets, wires) in the aqueous media using cetyltrimethylammonium bromide (CTAB) as a capping or stabilizing agent. They mentioned that a precise control of different reaction media and concentration of capping agent governs the overall morphology of copper telluride nanostructures.32 Cu(2−x)Te nanowires were magnificently synthesized by Dong and co-workers by using poly(vinylpyrrolidone) (PVP) as stabilizing agent.33
Surfactants have been broadly utilized as a template in the synthesis of semiconductor nanoparticles.34 Bis-quaternary ammonium surfactants represent a class of surfactants made up of two identical or different amphiphilic moieties having the structure of conventional (monomeric) surfactants connected by a spacer group. Because of a stronger hydrophobicity imparted by the twin hydrocarbon chains, such surfactants produce long threadlike structures in the millimolar concentration range. Higher hydrophobicity provides a driving force for stronger liquid/solid interfacial adsorption and that is imperative for size and shape controlled synthesis.35,36
Current research describes a simple hydrothermal route for the fabrication of copper telluride NPs in the presence of the aforementioned surfactants. Being one of the most eminent techniques for the synthesis of nanocrystals; the hydrothermal method exploits the solubility of almost all inorganic substances in water at elevated temperatures and pressure and the subsequent crystallization of the dissolved material from the fluid.37 At elevated temperatures water plays an essential role in the precursor material transformation because the structure of water at elevated temperature is different and the vapour pressure is much higher than that at room temperature. Various properties of the reactants, including their reactivity and solubility, also change at high temperature.38 The aforementioned properties deliver additional parameters to produce different high-quality nanoparticles, which are not possible at low temperatures. During the synthesis of nanocrystals, various parameters such as temperature, water pressure, reaction time, and the respective precursor–product system can be adjusted to maintain a high simultaneous nucleation rate and good size distribution. This method has several advantages over other growth processes such as use of simple equipment, low cost, large area uniform production, environmental friendliness and being less hazardous.39
Among the copper telluride NPs, examples of the hydrothermal synthesis method via bis-quaternary ammonium surfactants are rare. Thus, it is of much significance and necessity to explore a general and effective method for the fabrication of pure copper telluride nanoparticles. Herein, we report a convenient hydrothermal synthesis of bis-quaternary ammonium surfactant tailored copper telluride NPs with superior controllability in shape and size and with quantum confinement paving a promising way for exploiting their remarkable device applications. Preliminary studies for the CuTe NPs in the presence of a single surfactant have already been reported in our recent work and a probable chemical mechanism for the synthesis process was proposed in the same study.17 To compare the present work of copper telluride NPs prepared with three different surfactants in a detailed fashion, a few of the necessary results may reflect our previous work. Moreover, with a possible growth mechanism, the structural, optical and electrical properties of copper telluride nanoparticles are explored.40 Collective harnessing of the aforementioned properties allows for optimised absorption in photovoltaic and enhanced conductivity in thermoelectric devices. Herein, we also report the amended functional properties of the copper telluride NPs to be able to explore related and consequential applications in the near future.
2. Experimental details
2.1 Chemicals
Copper acetate, hydrazine, sodium telluride, 1-bromododecane, 1-bromotetradecane, 1-bromohexadecane, and N,N,N′,N′-tetramethylethylenediamine (TEMED) were all purchased from Sigma Aldrich and were used as received (>99%). All chemicals were used without further purification. The cationic surfactants dimethylenebis(dodecyldimethylammonium bromide) (12-2-12) (cmc = 0.84 mM), dimethylenebis(tetradecyldimethylammonium bromide) (14-2-14) (cmc = 0.16 mM), and dimethylenebis(hexadecyldimethylammonium bromide) (16-2-16) (cmc = 0.02 mM) were synthesized as reported in the literature.41–43 All surfactants were used after repeated recrystallization with acetone and ethyl acetate. In all preparation and rinsing stages, demineralized water was used.2.2 Preparation of Cu(2−x)Te
Copper telluride NPs were prepared through a reaction between copper acetate and sodium telluride in the presence of aqueous hydrazine. Typically, 10 mL of aqueous bis-quaternary ammonium surfactant solution [(12-2-12) (1 mM), (14-2-14) (0.5 mM) and (16-2-16) (0.25 mM)] containing 2.5% hydrazine was put in a conical flask. In another conical flask, 1 mM of copper acetate and 1 M NaOH were dissolved into 15 mL of distilled water. Both of the solutions were mixed and 10 mL of a 1 mM sodium telluride solution was added to this solution; the total solution was diluted to 40 mL under constant stirring for 10 minutes. The initial colour of the solution was light yellow, then it shifted to brown and then to greenish black within 5 min and then remained the same. The total solution was further transferred into a Teflon-lined autoclave with a volume capacity of 50 mL, up to 80% capacity of the total volume of the autoclave. The sealed autoclave was put into a 150 °C preheated furnace, and the reaction was kept for 32 h. After 32 h, the subsequent mixture was collected by centrifuging at 4500 rpm and washed several times with distilled water and ethanol.2.3 Characterization
UV-vis absorption spectra of the samples were obtained by using a UV spectrophotometer (Systronics 2202) in the range 200–900 nm. Photoluminescence (PL) spectra were measured by using an LS-55 (Perkin Elmer) instrument. These samples were all excited at 290 nm. Powder X-ray diffraction (PXRD) patterns were recorded on a Panalytical X’Pert Pro diffractometer using Cu Kα radiation. The copper telluride samples were scanned in the 2θ range from 10 to 80°. Different crystal planes and packing arrangements were determined with XRD. Electron microscopic studies for the shape and size of the prepared copper telluride nanoparticles were performed on a TECNAI 200 kV TEM (FEI, Electron Optics) operating at 100 kV. The TEM samples were prepared by sonication of the copper telluride nanoparticles in ethanol, placing a few microliters onto a carbon-coated copper grid. The grids were allowed to dry in the air. FTIR was measured by using an RZX (Perkin Elmer) A Fourier transform infrared (FTIR) spectrometer at room temperature in the range of 4000–400 cm−1 with the sample mould in KBr was used. Raman scattering measurements were performed at room temperature with a lab RAM HR system in a backscattering configuration. The instrument was calibrated to the same accuracy using silicon and a naphthalene standard.2.4 Electrical analysis of the nanoparticles
Electrical resistivity (ρ) of the copper telluride samples was measured by the standard two-probe method in a vacuum (Keithley-2611). A freshly prepared powder of Cu(2−x)Te nanoparticles was pressed into a round tablet under pressure at room temperature without using any organic lubricants.3. Results and discussion
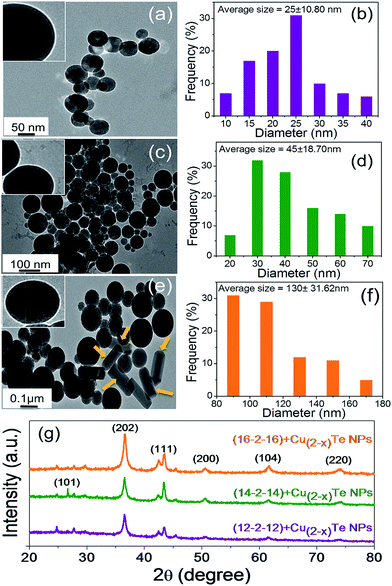
The powder XRD pattern of the as prepared copper telluride NPs is shown in Fig. 1g with characteristic features corresponding to the (101), (202), (111), (200), (104) and (220) planes. All the peaks are very prominent and relate to the hexagonal structure of crystalline copper telluride. The powder XRD data are consistent with the published XRD data.26 As expected, the peaks of copper telluride considerably broadened with the decreased size of the nanoparticles, confirmed by TEM observations as well. The size of the nanoparticles were estimated using Scherrer’s formula44 based on the full width at half-maximum (FWHM) of the different diffraction peaks, with different values (Table 1, ESI†).
 | (i) |
As shown in the figure, the XRD peaks of the different samples become progressively broader when the particle size decreases from (16-2-16) to (12-2-12) capped NPs. The full width at half-maximum (FWHM) of the (12-2-12) capped copper telluride nanoparticles was much broader than those of the (14-2-14) and (16-2-16) stabilized nanoparticles. From the different theta values, the average particle size for the copper telluride nanoparticles with (12-2-12), (14-2-14) and (16-2-16) surfactants was calculated to be about 15.24 nm, 28.76 nm and 34.69 nm, respectively. The XRD patterns illustrate that the pure copper telluride nanoparticles were obtained under the present synthetic conditions.
In order to explore the influence of the hydrocarbon chain length of the twin-tail surfactants on the shape and size of the copper telluride NPs, the microstructure of the copper telluride NPs was investigated by TEM. The spherical copper telluride NPs with (12-2-12), (14-2-14) and (16-2-16) at concentrations of 1 mM, 0.5 mM and 0.25 mM, respectively, are shown in Fig. 1 with successive increases in hydrocarbon chain length from top to bottom. We do not observe any appreciable change in the morphology for the (12-2-12) and (14-2-14) capped copper telluride NPs but the morphology of the NPs changed a little with the increase in chain length from 14 to 16, and the size of the copper telluride NPs dramatically increased with the increase in chain length (m = 12 to 16). For (12-2-12), spherical particles of 25 ± 10.8 nm in size were seen. As the hydrocarbon chain length approaches m = 14, 16 for (14-2-14) and (16-2-16) respectively, there is formation of large particles of 45 ± 18.7 nm and 130 ± 31.6 nm, respectively. This is suggestive of an increment in the average particle diameter with increasing tail length. Formation of some nanorods with (16-2-16) might be attributed to the long tails of surfactants and short spacers which lead to high solution viscosity at lower surfactant concentration. This behaviour has been ascribed to the formation of threadlike micelles from the surfactants, which leads to the formation of nanorods. Generally, the possibility of the formation of nanorods increases with increasing bis-quaternary ammonium surfactant alkyl chain length and these long tails have a preference to interact with each other via van der Waal interactions to form nanorod structures (shown by block arrows in Fig. 1e).45 A comprehensive elaboration of the present work is underway by increasing the tail length further to get desired features. Fig. 1b, d and f show histograms of the nanoparticles that correspond to the TEM images. The three different insets in Fig. 1a, c and e are evidently expressive of the presence of a bright monolayer indicating clear surfactant capping around each particle.
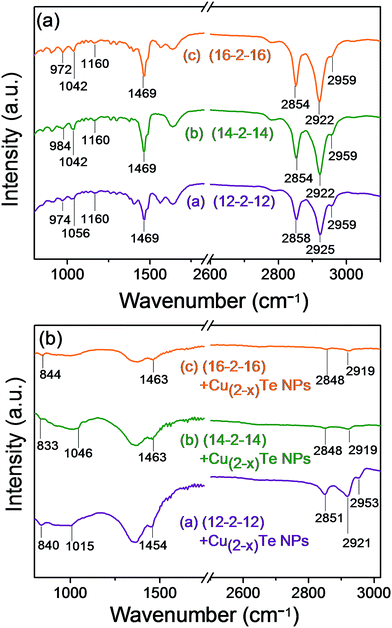
In order to substantiate the surface adsorption of the surfactants on the copper telluride NP surface, FTIR spectral studies of the NP samples and pure surfactants were carried out. Fig. 2a shows the representative FTIR spectra of the pure surfactants, that is, (12-2-12), (14-2-14) and (16-2-16) and with the copper telluride nanoparticles (Fig. 2b). The peaks at the higher frequency region of the pure bis-quaternary ammonium surfactants, that is (12-2-12), show symmetric and asymmetric stretching vibrations of methylene in the alkyl chains at 2858, 2925 and 2959 cm−1, which shift to 2851, 2921 and 2953 cm−1 in the case of the (12-2-12) capped copper telluride NPs. Symmetric and asymmetric stretching vibrations at 2854, 2922 and 2959 cm−1 shift to 2919 and 2848 cm−1 in the case of the (14-2-14) and (16-2-16) capped NPs. A scissoring mode of vibrations (δs(C–H)) at 1469 cm−1 for the pure surfactants shifts to 1454, 1463 and 1463 cm−1 for the (12-2-12) and (14-2-14) capped copper telluride NPs, respectively. The peaks at 1160, 1056, 1042, 984, 974 and 972 cm−1 in the case of the pure surfactants can be assigned to the ν(C–N+) stretching modes. These peaks shift to 1015 and 840 cm−1 for (12-2-12), 1046 and 833 cm−1 for (14-2-14) and 844 cm−1 for the (16-2-16) capped copper telluride nanoparticles and may be an indicator of higher energies of stretching vibrations corresponding to more gauche defects. Small vibrational frequency shifts were also detected for the bis-quaternary ammonium surfactant stabilized nanoparticles.46,47
 | ||
| Fig. 2 FTIR spectra of (a) pure surfactants and (b) Cu(2−x)Te NPs capped with (12-2-12), (14-2-14) and (16-2-16) surfactants. | ||
Hydrazine hydrate plays a critical role as a reducing agent as well as in the growth of telluride in a strong alkaline environment.48 On the other hand, hydrazine also combines with copper ions to form metal amides, which effectively suppress the formation of copper oxide. Copper amide reacts with active Te2− in solution to form the copper telluride nucleus under controlled hydrothermal conditions. The NP size at the nanoscale is accountable for the aforementioned advanced properties of these materials and nanostructured materials with respect to the bulk material are suitable for new applications. In the present work Cu(2−x)Te nanoparticles were synthesized with a diameter of 25 ± 10.8 nm, 45 ± 18.7 nm, and 130 ± 31.6 nm using (12-2-12), (14-2-14) and (16-2-16) surfactants, respectively.
Williamson–Hall analysis of the (12-2-12), (14-2-14) and (16-2-16) capped copper telluride nanoparticles was carried out by assuming three main models, i.e. the uniform deformation model (UDM), uniform stress deformation model (USDM), and uniform deformation energy density model (UDEDM). The Williamson–Hall method varies with tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ and does not follow the 1/cos
θ and does not follow the 1/cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ dependency in the Scherrer equation. Unaffected by small crystallite size, micro strain caused the reflection broadening.49 Depending on different θ positions the separation of size and strain broadening analysis was done using the Williamson–Hall method. The strain induced in the powders due to crystal imperfection and distortion was calculated using the following formula:
θ dependency in the Scherrer equation. Unaffected by small crystallite size, micro strain caused the reflection broadening.49 Depending on different θ positions the separation of size and strain broadening analysis was done using the Williamson–Hall method. The strain induced in the powders due to crystal imperfection and distortion was calculated using the following formula:
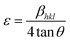 | (ii) |
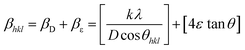 | (iii) |
 | (iv) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ was plotted with respect to 4
θ was plotted with respect to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
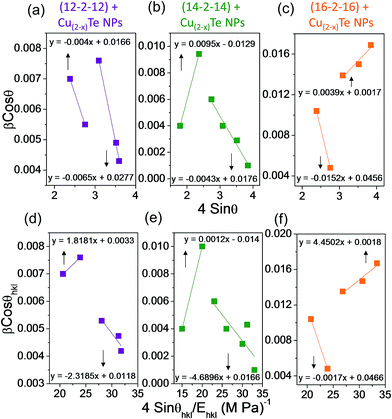
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ for the peaks of copper telluride NPs with different twin-tail surfactants. Strain and particles size were calculated from the slope and y-intercept of the fitted line, respectively. The plots exhibited a negative strain for the nanoparticles which may be caused by the lattice shrinkage that was observed in the calculation of the lattice parameters. The uniform deformation model analysis results for copper telluride NPs with various surfactants are shown in Fig. 3a–c. The altered plot of the Hall equation for copper telluride NPs indicates a positive and negative slope. The positive slope indicates the presence of tensile strain in the lattice, the negative slope was attributed to the presence of compressive strain. The strain is the manifestation of a dislocation network, and the decrease of strain indicates a decrease in concentration of lattice imperfections.50
θ for the peaks of copper telluride NPs with different twin-tail surfactants. Strain and particles size were calculated from the slope and y-intercept of the fitted line, respectively. The plots exhibited a negative strain for the nanoparticles which may be caused by the lattice shrinkage that was observed in the calculation of the lattice parameters. The uniform deformation model analysis results for copper telluride NPs with various surfactants are shown in Fig. 3a–c. The altered plot of the Hall equation for copper telluride NPs indicates a positive and negative slope. The positive slope indicates the presence of tensile strain in the lattice, the negative slope was attributed to the presence of compressive strain. The strain is the manifestation of a dislocation network, and the decrease of strain indicates a decrease in concentration of lattice imperfections.50
In the Uniform Stress Deformation Model (USDM), a generalized Hook’s law refers to the strain, keeping only the linear proportionality between stress and strain as given by σ = εEhkl, where σ is the stress of the crystal and Ehkl is the modulus of elasticity or Young’s modulus. This equation is an approximation that is valid for significantly small strain. Presuming the existence of small strain in the copper telluride NPs, Hook’s law can be used here. Applying the Hook’s law approximation to eqn (iv) yields:
 | (v) |
Young’s modulus, Ehkl for copper telluride has been reported as 115 GPa.51 The USDM plots were drawn with 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/Ehkl corresponding to the x-axis and β
θ/Ehkl corresponding to the x-axis and β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ to the y-axis for copper telluride NPs with different surfactants in Fig. 3d–f. Another model can be used to determine the energy density of a crystal and is called the uniform deformation energy density model (UDEDM). The crystals were assumed to have a homogeneous and isotropic nature. The energy density u (energy per unit volume) for an elastic system that follows Hook’s law can be calculated from u = ε2Ehkl/2. Then eqn (v) can be rewritten according to the energy and strain relation as below:
θ to the y-axis for copper telluride NPs with different surfactants in Fig. 3d–f. Another model can be used to determine the energy density of a crystal and is called the uniform deformation energy density model (UDEDM). The crystals were assumed to have a homogeneous and isotropic nature. The energy density u (energy per unit volume) for an elastic system that follows Hook’s law can be calculated from u = ε2Ehkl/2. Then eqn (v) can be rewritten according to the energy and strain relation as below:
 | (vi) |
The uniform deformation energy density model (UDEDM) can be calculated from the slope of the line plotted between βhkl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ versus 4
θ versus 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ(2u/Ehkl)1/2. The anisotropic energy density ‘u’ was calculated from the slope of these lines and the crystalline size D from the y-intercept. The comparison of the three results for copper telluride nanoparticles is possible from the analysis of Fig. 4a–c because the scattering of the points away from the linear expression is much less in Fig. 4 than Fig. 3. The crystalline size estimated from the y-intercept of the graphs from Fig. 3a–f and 4a–c are 9.2 nm (UDM), 13 nm (USDM) and 51 nm (UDEDM) for the (12-2-12) capped copper telluride NPs. For the (14-2-14) capped NPs, the crystalline size is 11.9 nm (UDM), 12.8 nm (USDM) and 81 nm (UDEDM), while for the (16-2-16) capped copper telluride NPs, the crystalline size was found to be 90.5 nm (UDM), 85.5 nm (USDM) and 50.6 nm (UDEDM).
θ(2u/Ehkl)1/2. The anisotropic energy density ‘u’ was calculated from the slope of these lines and the crystalline size D from the y-intercept. The comparison of the three results for copper telluride nanoparticles is possible from the analysis of Fig. 4a–c because the scattering of the points away from the linear expression is much less in Fig. 4 than Fig. 3. The crystalline size estimated from the y-intercept of the graphs from Fig. 3a–f and 4a–c are 9.2 nm (UDM), 13 nm (USDM) and 51 nm (UDEDM) for the (12-2-12) capped copper telluride NPs. For the (14-2-14) capped NPs, the crystalline size is 11.9 nm (UDM), 12.8 nm (USDM) and 81 nm (UDEDM), while for the (16-2-16) capped copper telluride NPs, the crystalline size was found to be 90.5 nm (UDM), 85.5 nm (USDM) and 50.6 nm (UDEDM).
Imperatively, the isotropic character of the line broadening is illustrated via the Williamsons–Hall plot, which indicates that the diffracting domains were isotropic and that there was also a microstrain contribution. The size–strain parameter may be attained from the “size–strain plot” (SSP). The data from the reflections at a high level have less importance and data precision is usually lower for the same. According to this estimation, it was assumed that the profile is fitted by a strain-contour via a Gaussian function and the crystalline size by a Lorentzian function.52 Accordingly, we have
 | (vii) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ versus (dhklβhkl
θ versus (dhklβhkl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ)2 and the data fitted to lines. The particle size was calculated from the slope and the root of the y-intercept elasticises the strain. The results obtained from the uniform deformation model (UDM), uniform stress deformation model (USDM), uniform deformation energy density model (UDEDM) and size–strain plot method are summarized in Table 3 of the ESI.†
θ)2 and the data fitted to lines. The particle size was calculated from the slope and the root of the y-intercept elasticises the strain. The results obtained from the uniform deformation model (UDM), uniform stress deformation model (USDM), uniform deformation energy density model (UDEDM) and size–strain plot method are summarized in Table 3 of the ESI.†
Raman spectroscopy involves the study of vibrational-rotational energy changes in molecules by means of scattering of light. Energy from the laser is exchanged with the molecules in such a way that the scattered light photons have a higher or lower energy than the incident photon. The distinction in energy is due to a shift in the rotational and vibrational energy of the molecules which gives information about the molecular energy levels. Raman spectroscopy is an important method for the determination of molecular structure, for locating functional groups in molecules or various chemical bonds and for the quantitative analysis of complex mixtures. In Raman scattering (Fig. 5a) each line has a characteristic polarization, which contributes additional information about the molecular structure.53 The peaks at 142.85 and 217.19 cm−1 are due to the translational and torsional motions of the molecules in lattice vibrations. The lattice vibrational modes of copper telluride nanoparticles were obtained at room temperature by Raman spectroscopy.54,55 Peaks at 413 and 521 cm−1 for the copper telluride nanoparticles are due to metal–surfactant vibrations and presumably due to the first overtone of the strongest band in the spectrum, respectively.56,57 Due to quantum confinement effects in the nanoparticles, the peaks at 142.85, 217.19, 413 and 521 cm−1 broaden due to the decreased particle size of the surfactant stabilized copper telluride nanoparticles. The overall change in Raman spectra with particle size due to all of these effects is shown in Fig. 5a. There is also a decent level of agreement with the peak width when strain is considered. Although, when the effects of confinement and defects are included, the aforesaid agreement is good, but less perfect. Overall, it is thought that strain also plays the most significant role. Together, strain and confinement can account for much of the broad and asymmetric character of the observed peaks.58,59 Comparison of Raman spectra suggests that the band intensities relatively decrease as the particle size decreases and the results are in accordance with the available literature.
 | ||
| Fig. 5 (a) Raman and (b) photoluminescence spectra of the (12-2-12), (14-2-14) and (16-2-16) capped copper telluride NPs. | ||
The room temperature photoluminescence (PL) spectra of copper telluride nanoparticles were measured at an excitation wavelength of 290 nm. Usually for semiconductor nanoparticles, two emission peaks can be observed – exciton luminescence and trapped luminescence.60 Fig. 5b shows the broad PL emission spectra of the (12-2-12), (14-2-14) and (16-2-16) stabilized copper telluride NPs; the emission peaks at 335.10, 337.12 and 338.22 nm, respectively, are attributed to blue emission and are evidence of the quantum confinement effect, with decreasing nanoparticle size. Another emission peak at around 652.54 nm observed in all the copper telluride samples may be attributed to green emission in the visible region. The green emission peak at around 652.54 nm is a defect related emission peak and is generated from deep-level or trap state emission.61 The broadening of the PL emission peaks is observed as the diameter of the nanoparticles decreases for the (16-2-16), (14-2-14) and (12-2-12) capped NPs respectively. The PL emission from the nanoparticles is strongly affected by strain, which results in shifting and broadening of the PL peaks. This broadening of the PL peak can be accredited to strain in the nanoparticles caused by size induced quantization effects. The broadening of the PL band may also be caused by the broadening of the band edges due to variations generated by the high concentration of defects or impurities. The defects or strain/stress are introduced due to confinement effects at the nanoscale regime.62 The copper telluride NPs grown by the hydrothermal method exhibit broad emission PL spectrum peaks at 335.10, 337.12 and 338.22 nm for the different nanoparticles.
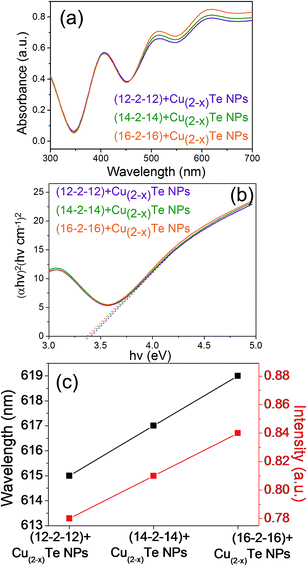
Fig. 6a demonstrates the absorption spectra of copper telluride nanoparticles capped with altered bis-quaternary ammonium surfactants (12-2-12, 14-2-14 and 16-2-16), a blue-shift is observed, which becomes stronger for decreasing particle size (Fig. 6c). To examine the quantum confinement effect of the synthesized nanoparticles, UV-vis absorption spectroscopy was employed. The peaks in the UV absorption spectra are indicative of the band gap of the copper telluride nanoparticles. The optical band gap energy of the nanoparticles was calculated using the Tauc relation:63
| αhv = B(hv − Eg)n | (viii) |
The optical band gap was determined by extrapolating the straight portion of the curve between (αhv)2 and hv. The optical band gap values of the as prepared (12-2-12), (14-2-14) and (16-2-16) capped copper telluride NPs were evaluated as 3.42 eV, 3.40 eV and 3.37 eV respectively (Fig. 6b). The evaluated band gap values are larger than the direct band gap of bulk copper telluride (1.1 to 1.7 eV), precisely illustrating quantum confinement. Quantum confinement can be observed once the diameter of a material is of the same magnitude as the de Broglie wavelength of the electron wave function. When materials are so small, their electronic and optical properties deviate substantially from those of the bulk materials. As the confining dimension decreases and reaches a certain limit, typically at the nanoscale, the energy spectrum becomes discrete and consequently, the band gap becomes size-dependent. This ultimately results in a blue shift as the size of the particles decreases as observed earlier. Both the absorption and PL spectra shown in Fig. 6c and 5b exhibit a “quantum size effect”. The excitonic peak position in the absorption spectra shifts a little towards a higher wavelength when the particle size increases for the (12-2-12), (14-2-14) and (16-2-16) stabilized nanoparticles. In the meantime, the PL peak also shifts position at 335.10, 337.12 and 338.22 nm for the (12-2-12), (14-2-14) and (16-2-16) stabilized nanoparticles which shows the characteristic property of quantum confinement.
To probe the bis-quaternary ammonium surfactant capped copper telluride nanoparticles further, the samples were subjected to electrical conductivity measurements at room temperature. Electrical conductivity is an important factor in discovering information about transport phenomena. Recent works on the electrical properties of semiconductor nanoparticles have paid considerable attention toward the conduction mechanism.64 Changes in the size of electrically conducting materials can change the magnitude of electrical conductivity; apparently the particle size plays a crucial role in conductivity.65,66 Fig. 7a–c show the linear current–voltage (I–V) curves, which are virtually symmetric about the origin, implying that the contacts are ohmic. The electrical resistivity (Fig. 7d) values for the (12-2-12), (14-2-14) and (16-2-16) capped copper telluride nanoparticles vary for the different copper telluride nanoparticles from 78.31 to 11.49 Ω cm, 105.18 to 21.34 Ω cm and 44.04 to 21.43 Ω cm, respectively, at different temperatures (293–373 K). On the basis of size including the length, width and thickness of the samples, the electrical conductivities of the different samples were calculated to be 0.012–0.170 S cm−1, 0.018–0.055 S cm−1 and 0.023–0.053 S cm−1 for the (12-2-12), (14-2-14) and (16-2-16) capped copper telluride NPs, respectively.67 Copper telluride nanoparticles have good conductivity with increasing temperature which metal conductance an increase in conductivity of the NPs with temperature reveals semiconductor behaviour.68 Unfortunately, since there are few standard values available for the electrical conductivity of Cu(2−x)Te nanoparticles in the literature, we must mainly compare within our present experimental results after repeated experiments. Wang and co-workers reported an increase in electrical conductivity with rises in temperature for different structures (nanoparticles, nanosheets and nanobelts). The electrical conductivity of nanosheets was less than that for nanobelts and nanoparticles were reported as the least conductive.69 The observations can also be compared with the studies carried out by Dong et al., who calculated the electrical conductivity of Cu(2−x)Te nanowires at room temperature to be 5.99 × 103 Ω−1 m−1.32 The resistivity values postulated by Yang et al. for Cu(2−x)Te films were reported as 1.78 and 11.78 Ω cm, prior and subsequent to the completion of reduction, respectively.70 In our experiment, the observed values of conductivity at room temperature for bis-quaternary ammonium surfactant stabilized copper telluride nanoparticles were found to be greater as compared to previous reported results.71 In addition, the rich behaviour introduced by the conductivity in copper telluride nanoparticles makes these materials a topic of ongoing interest.
 | ||
| Fig. 7 (a–c) I–V characteristics at different temperatures and (d) variation of conductivity with temperature for (12-2-12), (14-2-14) and (16-2-16) stabilized copper telluride NPs. | ||
Because of the hydrophobic character of the various surfactants in this study, it is imperative to discuss this character with respect to the morphological impacts of the NPs. Bis-quaternary ammonium surfactants have attracted much attention due to their unique bulk and interfacial properties, such as lower critical micelle concentration, higher interfacial activity, etc., as compared to their monomers and conventional surfactants.72 In the present work, we used bis-quaternary ammonium surfactants with a small spacer length, i.e. s = 2. The two head groups are consistently drawn close and thus the absorbed molecules are packed more tightly than the single chain and conventional molecules.73 For systems involving the above mentioned surfactants, the size as well as morphology can be controlled and control of the nanoparticle shape and size results in excellent properties for third generation devices. An overview of the TEM images indicates that the twin-tail surfactants appear to be the main shape directing agents with well-defined geometry of copper telluride nanoparticles in comparison to the previously reported results for the same nanoparticles with conventional surfactants such as cetyltrymethylammonium bromide (CTAB), polyvinylpyrrolidone (PVP), dipropylamine (DPrA), etc.32,33,69
On the basis of critical micelles concentration range, (16-2-16) was found to be more hydrophobic in comparison to the (14-2-14) and (12-2-12) surfactants.74 There was a little morphological change only for the (16-2-16) capped copper telluride NPs, but a change in intensity of the (202) diffraction peak was observed with different surfactants. The intensity of the (202) diffraction peak increases with increasing hydrophobicity of the surfactants as (12-2-12), (14-2-14) and (16-2-16), respectively. Greater hydrophobicity delivers a better capping ability and stronger liquid–solid interfacial adsorption. The replacement of surfactants from (12-2-12) to (16-2-16) in the case of the copper telluride NPs consequently led to an increase in size with the increasing hydrophobicity of the surfactant.
4. Conclusions
An assortment of bis-quaternary ammonium surfactants were engaged in the synthesis of copper telluride nanoparticles in a single reaction at 150 °C. TEM images confirmed the spherical morphology and size of the nanoparticles with different surfactants. Hydrophobicity was observed to be the main contributing parameter for the size controlled synthesis of copper telluride nanoparticles. The average diameter of these nanoparticles are 25 ± 10.8, 45 ± 18.7 and 130 ± 31.6 nm for (12-2-12), (14-2-14) and (16-2-16), respectively. The UV-vis studies reveal that the optical band gap increases as the size of the nanoparticles decreases which shows quantum confinement. The optical band gap energy was determined to be 3.42, 3.40 and 3.37 eV for 25 ± 10.8, 45 ± 18.7 and 130 ± 31.6 nm copper telluride nanoparticles, respectively. The PL emission peaks at 335.10, 337.12 and 338.22 nm for the different nanoparticles ascribed to blue emission were observed for the copper telluride nanoparticles, another emission peak at around 652.54 nm was also observed and attributed to green emission in the visible region. The electrical conductivities of the different samples were measured to be 0.012–0.170 S cm−1, 0.018–0.055 S cm−1 and 0.023–0.053 S cm−1 for the (12-2-12), (14-2-14) and (16-2-16) capped copper telluride NPs, respectively. Therefore, this study describes the facile synthesis of shape and size controlled copper telluride nanoparticles with peculiar ammonium surfactants. Finally, the suitability of these nanoparticles for various interesting applications especially in solar cells, optical filters, super ionic materials, etc., can be understood on the basis of these properties. Steered by the afore-discussed properties, further elaboration of various consequential applications is underway.Acknowledgements
The authors are indebted to the Shoolini University of Biotechnology & Management Sciences, Solan, India for providing basic laboratory.Notes and references
- K. Yu, S. Singh, N. Patrito and V. Chu, Langmuir, 2004, 20, 11161 CrossRef CAS PubMed.
- S. Kango, S. Kalia, P. Thakur, B. Kumari and D. Pathania, Adv. Polym. Sci., 2015, 267, 283 CrossRef CAS.
- P. Reiss, M. Protiere and L. Li, Small, 2009, 5, 154 CrossRef CAS PubMed.
- G. F. Brown and J. Q. Wu, Laser Photonics Rev., 2009, 3, 394 CrossRef CAS.
- R. T. Ross and A. J. Nozik, J. Appl. Phys., 1982, 53, 3813 CrossRef CAS.
- T. Nann and W. M. Skinner, ACS Nano, 2011, 5, 5291 CrossRef CAS PubMed.
- S. Kolodinski, J. H. Werner, T. Wittchen and H. J. Queisser, Appl. Phys. Lett., 1993, 63, 2405 CrossRef CAS.
- I. Honma, S. Hirakawa, K. Yamada and J. M. Bae, Solid State Ionics, 1999, 118, 29 CrossRef CAS.
- P. Raizada, P. Singh, A. Kumar, B. Pare, S. B. Jonnalgadda and P. Thakur, Appl. Catal., A, 2014, 486, 159 CrossRef CAS.
- M. S. Bakshi, P. Thakur, S. Sachar and T. S. Banipal, Mater. Lett., 2007, 61, 3762 CrossRef CAS.
- X. Wu, J. Zhou, A. Duda, Y. Yan, G. Teeter, S. Asher, W. K. Metzger, S. Demtsu, S. H. Wei and R. Noufi, Thin Solid Films, 2007, 515, 5798 CrossRef CAS.
- J. Zhou, X. Wu, A. Duda, G. Teeter and S. H. Demtsu, Thin Solid Films, 2007, 515, 7364 CrossRef CAS.
- M. Kobayashi, K. Ishikawa, F. Tachibana and H. ErratumOkazaki, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 38, 3050 CrossRef CAS.
- S. Kashida, W. Shimosaka, M. Mori and D. Yoshimura, J. Phys. Chem. Solids, 2003, 64, 2357 CrossRef CAS.
- P. Singh, P. Raizada, S. Kumari, A. Kumar, D. Pathania and P. Thakur, Appl. Catal., A, 2014, 476, 9 CrossRef CAS.
- B. A. Mansour, B. S. Farag and S. A. Khodier, Thin Solid Films, 1994, 247, 112 CrossRef CAS.
- D. Jamwal, G. Kaur, P. Raizada, P. Singh, D. Pathak and P. Thakur, J. Phys. Chem. C, 2015, 119, 5062 CAS.
- O. Palchik, R. Kerner, Z. Zhu and A. Gedanken, J. Solid State Chem., 2000, 154, 530 CrossRef CAS.
- L. Z. Zhang, Z. H. Ai, F. L. Jia, L. Liu, X. L. Hu and J. C. Yu, Chem.–Eur. J., 2006, 12, 4185 CrossRef CAS PubMed.
- B. Lim, Y. J. Xiong and Y. N. Xia, Angew. Chem., Int. Ed., 2007, 46, 9279 CrossRef CAS PubMed.
- Y. Zhang, Z. P. Qiao and X. M. Chen, J. Mater. Chem., 2002, 12, 2747 RSC.
- X. Wu, J. Zhou, A. Duda, Y. Yana, G. Teeter, S. Asher, W. K. Metzger, S. Demtsu, S. H. Wei and R. Noufi, Thin Solid Films, 2007, 515, 5798 CrossRef CAS.
- Q. Wang, G. Chen, X. R. Shi, R. C. Jin, L. Wang and D. H. Chen, Powder Technol., 2011, 207, 192 CrossRef CAS.
- L. Wenhua, R. Zamani, P. R. Gil, B. Pelaz, M. Ibanez, D. Cadavid, A. Shavel, A. R. Alvarez-Puebla, W. J. Parak, A. Jordi and A. Cabot, J. Am. Chem. Soc., 2013, 135, 7098 CrossRef PubMed.
- B. Li, Y. Xie, J. X. Huang, H. L. Su and Y. T. Qian, J. Solid State Chem., 1999, 146, 47 CrossRef CAS.
- B. Li, Y. Xie, J. X. Huang, Y. Liu and Y. T. Qian, Chem. Mater., 2000, 12, 2614 CrossRef CAS.
- P. Kumar and K. Singh, Cryst. Growth Des., 2009, 9, 3089 CAS.
- Y. Zhang, Z. P. Qiao and X. M. Chen, J. Mater. Chem., 2002, 12, 2747 RSC.
- B. S. Farag and S. A. Khodier, Thin Solid Films, 1991, 201, 231 CrossRef CAS.
- W. Z. Wang, Y. Geng, P. Yan, F. Y. Liu, Y. Xie and Y. T. Qian, Inorg. Chem. Commun., 1999, 2, 83 CrossRef CAS.
- X. J. Zhang, D. E. Zhang, X. M. Ni and H. G. Zheng, Solid State Commun., 2006, 139, 412 CrossRef CAS.
- L. Zhang, Z. Ai, F. Jia, L. Liu, X. Hu and J. C. Yu, Chem.–Eur. J., 2006, 12, 4185 CrossRef CAS PubMed.
- G. H. Dong, Y. J. Zhu, G. F. Cheng and Y. J. Ruan, Mater. Lett., 2012, 76, 69 CrossRef CAS.
- Y. Shen, J. Bao, N. Dai, J. Wu, F. Gu, J. C. Tao and J. C. Zhang, Appl. Surf. Sci., 2009, 255, 3908 CrossRef CAS.
- A. Bernheim-Goswasser, R. Zana and Y. Talmon, J. Phys. Chem. B, 2000, 104, 4005 CrossRef.
- M. S. Bakshi and G. Kaur, J. Colloid Interface Sci., 2005, 289, 551 CrossRef CAS PubMed.
- C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025 CrossRef CAS PubMed.
- C. Y. Lee, T. Y. Tseng, S. Y. Li and P. Lin, J. Appl. Phys., 2006, 99, 024303 CrossRef.
- L. Zhang, Z. Ai, F. Jia, L. Liu, X. Hu and J. C. Yu, Chem.–Eur. J., 2006, 12, 4185 CrossRef CAS PubMed.
- S. Singh, P. Liu, A. Singh, C. Coughlan, J. Wang, M. Lusi and K. M. Ryan, Chem. Mater., 2015, 27(13), 4742 CrossRef CAS .
- G. Bai, J. Wang, H. Yan, Z. Li and R. K. Thomas, J. Phys. Chem. B, 2001, 105, 3105 CrossRef CAS.
- R. Zana, M. Benrraou and R. Rueff, Langmuir, 1991, 7, 1072 CrossRef CAS.
- S. D. Wettig and R. E. Verrall, J. Colloid Interface Sci., 2001, 244, 377 CrossRef CAS.
- J. P. Borah, J. Barman and K. C. Sharma, Chalcogenide Lett., 2008, 5, 201 CAS.
- T. Jain, A. R. Tehrani-Bagha, H. Shekhar, R. Crawford, E. Johnson, K. Norgaard, K. Holmberg, P. Erhartc and K. Moth-Poulsen, J. Mater. Chem. C, 2014, 2, 994 RSC.
- C. Naselli, J. F. Rabolt and J. D. Swalen, J. Chem. Phys., 1985, 82, 2136 CrossRef CAS.
- L. X. Zhang, X. P. Sun, Y. H. Song, X. Jiang, S. J. Dong and E. K. Wang, Langmuir, 2006, 22, 2838 CrossRef CAS PubMed.
- B. Wan, C. Hu, W. Zhou, H. Liu and Y. Zhang, Solid State Sci., 2011, 13, 1858 CrossRef CAS.
- A. Z. Khorsand, W. H. A. Majid, M. E. Abrishami and R. Yousefi, Solid State Sci., 2011, 13, 251 CrossRef.
- D. Chrastina, G. M. Vanacore, M. Bollani, P. Boye, S. Schoder, M. Burghammer, R. Sordan, G. Isella, M. Zani and A. Tagliaferri, Nanotechnology, 2012, 23, 155702 CrossRef CAS PubMed.
- V. J. Fulari, V. P. Malekar and S. A. Gangawane, Prog. Electromagn. Res., 2010, 12, 53 CrossRef.
- N. S. Goncalves, J. A. Carvalho, Z. M. Lima and J. M. Sasaki, Mater. Lett., 2012, 72, 36 CrossRef CAS.
- R. J. H. Clark and T. J. Dines, Angew. Chem., Int. Ed. Engl., 1986, 25, 131 CrossRef.
- S. S. Dhasade, S. H. Han and V. J. Fulari, J. Semicond., 2012, 33, 093002 CrossRef.
- B. J. Park, H. S. Seo, J. T. Ahn, J. H. Song, W. J. Chung and D. Y. Jeon, J. Mater. Res., 2011, 26, 2394 CrossRef CAS.
- B. Minceva-Sukarovaa, M. Najdoskia, I. Grozdanova and C. J. Chunnilallb, J. Mol. Struct., 1997, 410, 267 Search PubMed.
- A. Khare, B. Himmetogu, M. Johnson, D. J. Norris, M. Cococcioni and E. S. Aydil, J. Appl. Phys., 2011, 111, 083707 CrossRef.
- A. Tanaka, S. Onari and T. Arai, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 45(12), 6587 CrossRef CAS.
- W. H. Weber, K. C. Bass and J. R. McBride, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 178 CrossRef CAS.
- A. Fasoli, A. Colli, F. Martelli, S. Pisana, P. H. Tan and A. C. Ferrari, Nano Res., 2011, 4, 343 CrossRef CAS.
- S. Kumar, V. Singh, A. Vohra and S. K. Chakarvarti, Am. J. Mater. Sci. Technol., 2013, 1, 74 Search PubMed.
- A. Z. Martijn, Nanoscale, 2012, 4, 3711 RSC.
- J. Tauc, R. Grigorovici and A. Vancu, Phys. Status Solidi B, 1996, 15, 627 CrossRef.
- M. R. Dirmyer, J. Martin, G. S. Nolas, A. Sen and J. V. Badding, Small, 2009, 5, 933 CrossRef CAS PubMed.
- Y. B. Yi, Acta Mater., 2008, 56, 2810 CrossRef CAS.
- B. A. Mansour, B. S. Farag and S. A. Khodier, Thin Solid Films, 1994, 247, 112 CrossRef CAS.
- Y. Son, M. Park, Y. Son, J.-S. Lee, J.-H. Jang, Y. Kim and J. Cho, Nano Lett., 2014, 14, 1005 CrossRef CAS PubMed.
- L. D. Zhao, B. P. Zhang, W. S. Liu and J. F. Li, J. Appl. Phys., 2009, 105, 023704 CrossRef.
- Q. Wang, G. Chen, D. Chen and R. Jin, CrystEngComm, 2012, 14, 6962 RSC.
- H.-J. Yang, C.-Y. Chen, F.-W. Yuan and H.-Y. Tuan, J. Phys. Chem. C, 2013, 117, 21955 CAS.
- H. M. Pathan, C. D. Lokhande, D. P. Amalnerkar and T. Seth, Appl. Surf. Sci., 2003, 218, 290 CrossRef CAS.
- R. Zana, Adv. Colloid Interface Sci., 2002, 97, 205 CrossRef CAS PubMed.
- K. Esumi, M. Iitaka and Y. Koide, J. Colloid Interface Sci., 1998, 208, 178 CrossRef CAS PubMed.
- M. S. Bakshi, P. Thakur, P. Khullar, G. Kaur and T. S. Banipal, Cryst. Growth Des., 2010, 10, 1813 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra24396c |
| This journal is © The Royal Society of Chemistry 2016 |