Alginate-assisted enrichment and purification of mesenchymal stem cells†
Abstract
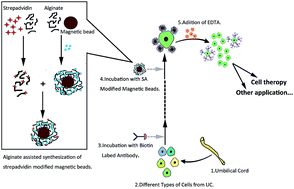
The umbilical cord contains different populations of stem/progenitor cells such as mesenchymal, hematopoietic, trophoblastic and endothelial cells. However, the isolation methods currently in use are different, and the resulted cells are liable to cross contamination. Thus it is increasingly difficult to compare and contrast the study outcomes, which hinders progress in the field of stem cell applications. In an attempt to address this issue, we have used a magnetism assisted self-assembly method for cell capturing and releasing in combination with antibodies to mesenchymal stem cell antigens, CD105, CD73 and CD90, to identify and localize stem cells within the placenta umbilical cord. This approach is capable of detecting a minimum of 10 000 mesenchymal stem cells, and has also shown excellent specificity to distinguish other types of cells.

 Please wait while we load your content...
Please wait while we load your content...