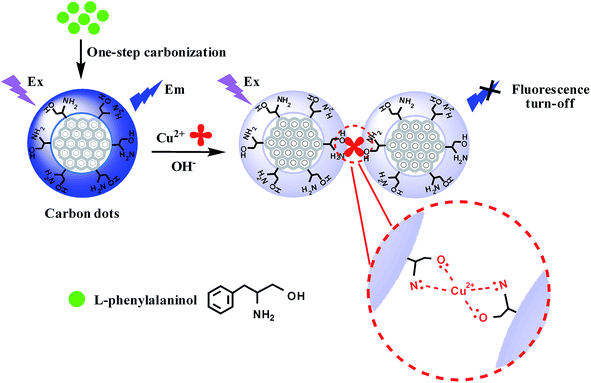
One-step facile synthesis of novel β-amino alcohol functionalized carbon dots for the fabrication of a selective copper ion sensing interface based on the biuret reaction†
Xi Wang,
Xin Shen,
Bingzhi Li,
Guoyi Jiang,
Xuemin Zhou* and
Huijun Jiang*
School of Pharmacy, Nanjing Medical University, No. 818 Tianyuan East Road, Nanjing 211166, Jiangsu, P. R. China. E-mail: xueminzhou001_001@hotmail.com; huijun_jiang2006@hotmail.com; Fax: +86 25 86868476; Tel: +86 25 86868476
First published on 9th February 2016
Abstract
In this paper, a facile and one-step hydrothermal carbonization approach has been developed for the first time to synthesize β-amino alcohol functionalized fluorescent carbon dots (CDs) with L-phenylalaninol as the sole precursor source. The present CDs with β-amino alcohol moieties attached to the surface were successfully applied to fabricate a novel, simple fluorescent sensing interface for selective detection of copper ions under basic conditions based on the biuret reaction. The added copper ions, not only lead to the specific cross-linked formation of the violet complex with the two β-amino alcohol units on the surface of the CDs, but also in turn lead to the aggregation of the functionalized CDs and induce fluorescence quenching. Such fluorescence responses can be used for monitoring copper ions in the range of 0.01–100 μmol L−1 under basic conditions with the limit of detection (LOD) as low as 3.2 nmol L−1. Moreover, the proposed fluorescent sensor can resist interferences from other metal ions and can be employed for the detection of copper ions in water samples. Due to the simplicity and effectiveness, it exhibits great promise as a practical sensing platform for copper ions especially under basic conditions.
1. Introduction
Carbon based dots, including graphene quantum dots (GQDs) and carbon dots (CDs) are a new form of zero-dimensional carbonaceous nanomaterials.1,2 CDs are recently discovered nanocarbons that comprise discrete, quasi-spherical carbon nanoparticles with sizes less than 10 nm.3,4 The typical structure of CDs is a core–shell structure consisting of oxygen/nitrogen-based groups or other chemical groups as the shell and sp2 hybridized amorphous or nanocrystalline carbon as the core.5 Compared with organic dyes and traditional semiconductor quantum dots, CDs have strong fluorescence emission in the visible spectral range, excellent water solubility, low toxicity, fine resistance to photobleaching, as well as ease of synthesis and surface modification, etc.6,7 Because of these unique properties, CDs can be employed for many applications such as in biosensing, bioimaging, drug delivery, especially in fluorescent sensors by monitoring the changes of fluorescence intensity under external physical or chemical stimuli. To obtain fluorescent CDs with adequate quantum yields (QYs), polymer passivation8 or chemical functionalization of their surfaces9 during or after synthesis is often needed. However, these procedures are often complicated by time-consuming multiple steps and lead to increased particle sizes of CDs, thus imposing limitations on the applications.10 Recently, the in situ one-step synthesis of CDs has been developed by means of heteroatom doping without any further surface modification (using two precursors in one-pot route)11 or only one precursor without using any surface passivating agents12 to obtain unique fluorescent performance.Copper ions (Cu2+), the third most abundant transition metal ions except zinc and iron ion in human body, play a critical role not only in electron transfer processes of many biological reactions,13 but also in the areas of environmental, and chemical systems.14 Many brain diseases, such as Alzheimer's, Parkinson's disease, are related to Cu2+.15 Due to its non-biodegradability and accumulation in the environment,16 Cu2+ can be toxic at elevated concentrations, although it is an essential micronutrient to aquatic organisms.17 In recent years, several studies have shown that relatively low levels of Cu2+ may cause oxidative stress in marine cnidarians.18 What's more, Cu2+ is listed as priority pollutant by the U. S. Environmental Protection Agency (EPA) and the limit of it in drinking water set by EPA is 1.3 ppm (∼20 μmol L−1).19 Therefore, with the purpose of controlling Cu2+ pollution and Cu2+-induced toxic effect, there is a great demand for a simple, green, reliable and sensitive strategy to qualify and quantify the level of Cu2+ presented in the environment and the ecosystem. The traditional detection methods for Cu2+ including atomic absorption spectrometry (AAS),20 electrochemical method,21 colorimetric method22 and inductively coupled plasma mass spectrometry (ICPMS).23 It is more facile to quantitatively determine copper ions by fluorescent methods without complicated operations or costly instruments, meanwhile, this can also be simply achieved by monitoring the change of the fluorescence intensity. For the previous fluorescence sensing systems, the detection was proceeded almostly under neutral or weak acidic conditions. Under basic conditions, Cu2+ is unstable due to its hydrolysis,24 and therefore, only a limited range of pH is suitable for the detection of Cu2+.
It has been recognized for more than a century that proteins, peptides and a variety of other compounds can coordinate with copper ions under basic conditions, which has been termed as the biuret reaction. Nowadays, the biuret test is still used for the UV-vis measurement of proteins and peptides in clinical laboratory. During the biuret reaction, the violet 5- or 6-member ring complexes were formed by copper(II) cross-chelating with nitrogen (amino nitrogen, amide nitrogen, etc.) or oxygen (hydroxyl oxygen, carboxylic oxygen etc.) as the coordination atoms.25 Carunchio's group reported the complex formation between copper(II) and several β-amino alcohols in aqueous solution and found that β-amino alcohols could be coordinated easily with copper(II), rather than other mental ions, to form stable 5-member chelate rings under basic conditions.26 However, the fluorescent detection of Cu2+ based on this kind of biuret reaction has not been reported yet.
In this article, we firstly designed and synthesized β-amino alcohol functionalized fluorescent CDs by a facile one-step hydrothermal carbonization using L-phenylalaninol as the sole precursor for the first time, and used them to fabricate a novel fluorescence sensing interface for selective detection of copper ions under basic conditions based on the biuret reaction (Scheme 1). The proposed fluorescent sensor can resist the interferences from other metal ions and can be employed for the detection of copper ions in water samples.
2. Experimental section
2.1 Materials
L-Phenylalaninol, quinine sulfate, dialysis membranes (regenerated cellulose, molecular weight cut off (MWCO) 500) are purchased from Aladdin reagent (Shanghai, China). The phosphate buffered solution (PBS) with different pH values are prepared by adding a concentrated phosphoric acid solution or sodium hydroxide solution into a 0.01 mol L−1 PBS at pH 7.4. Deionized water (DI, 18 MΩ) is produced by a water purification system (Milli-Q®, Millipore, Milford, MA, USA) water purification system and used in all experiments.2.2 Instruments and characterizations
Fluorescence spectra were recorded on a HITACHI F-4600 (Japan) fluorescence instrument. UV-vis spectra were obtained on a Shimadzu UV-2450 spectrophotometer. The transmission electron microscopic (TEM) and high-resolution transmission electron microscopic (HRTEM) images were acquired on a JEOL JEM-2100 (HR) transmission electron microscopy (Tokyo, Japan). FT-IR spectra were obtained by TENSOR 27. Raman spectra were performed by using a DXR Smart Raman Spectrometer excited with 633 nm laser radiation (Thermo Scientific, USA). X-ray photoelectron spectroscopy (XPS) spectra were collected in a PHI Quantera II spectrometer (Japan) and samples were freeze dried before XPS measurements.2.3 Synthesis of the β-amino alcohol functionalized CDs
The CDs were prepared by a modified hydrothermal carbonization method.27 Briefly, 0.05 g L-phenylalaninol was dispersed in 5 mL deionized water, followed by adjusting the pH to 9.0 by adding NaOH solution (0.1 mol L−1), and then treated by ultrasound for 30 min to obtain uniform suspension. The suspension was sealed into the Teflon-lined autoclave chamber and then treated by nitrogen gas for 20 min to remove dissolved O2 followed by hydrothermal treatment at 180 °C for 10 h. After cooling to room temperature, 10 mL deionized water was added to obtain the CDs solution. Then, it was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min to remove large precipitates. For further purifying the CDs, the resultant solution was dialyzed in a dialysis bag for 24 h (MWCO: 500). The purified CDs were preserved at room temperature for further use.
000 rpm for 20 min to remove large precipitates. For further purifying the CDs, the resultant solution was dialyzed in a dialysis bag for 24 h (MWCO: 500). The purified CDs were preserved at room temperature for further use.
2.4 Measurement of Cu2+
The mixed solution for fluorescence sensing is prepared via 800 μL of PBS (0.01 mol L−1, pH = 10.5) and 100 μL of CDs (2.85 mg mL−1). Different amounts of Cu2+ were added and shaken thoroughly at room temperature. The mixtures were diluted to 1 mL with deionized water and mixed thoroughly. 500 seconds later, the fluorescence spectra were recorded by the fluorescence spectrophotometer excited at 340 nm (λex = 340 nm).2.5 Selectivity experiments
The fluorescence and UV-vis responses of the CDs to the selected metal ions were examined by a similar procedure used above for Cu2+. The CDs and a series of metal ions (100 μmol L−1 Na+, K+, Ca2+, Mg2+; 20 μmol L−1 Fe2+ and Fe3+) were mixed with 800 μL of PBS (0.01 mol L−1, pH = 10.5), respectively. The mixtures were then added into a spectrophotometer quartz cuvette and the spectra were then measured at λex = 340 nm.2.6 Analysis of water samples
The water samples included lake water (Xuanwu Lake, Nanjing) and tap water. The lake water was filtered to remove solid suspensions using the 0.45 μm membranes while the tap water was directly used. The fluorescent sensing interface was applied to detect Cu2+ in water samples spiked with different concentrations of Cu2+, and the resultant fluorescence spectra were measured under the conditions identical to those described above.3. Results and discussion
3.1 Synthesis and characterization of the β-amino alcohol functionalized CDs
Many methods have been proposed to prepare CDs during last decade and can be roughly classified into “Top-down” and “Bottom-up” approaches.28,29 Bottom-up organic approaches include carbonization of carbohydrates,30 self-assembly of polycyclic aromatic hydrocarbons (PAHs),31 and organic synthesis from small molecules, etc.32 In this work, L-phenylalaninol was used as the sole precursor to synthesize CDs through an elegantly simple one-pot hydrothermal method without any further modification or surface passivation. Compared with the conventional two-step synthesis of carbon dots, this strategy of “synthesis-modification integration”33 is simpler and more efficient. (1) Basically, the benzene ring in L-phenylalaninol is a good carbon source due to the high carbon content. (2) The amino group in L-phenylalaninol guarantee that the synthetic product is nitrogen-doped CDs, which could significantly improve the QY of CDs.34,35 The prepared CDs exhibited excellent water-solubility compared with water-insoluble L-phenylalaninol. During the synthesis procedure, different hydrothermal reaction time (4, 6, 8, 10, 12, and 14 h) was investigated and the QYs were compared (Table S1 and Fig. S1 in the ESI†). The detailed measurement methods were stated in the ESI.† The CDs hydrothermal 10 h had the highest QY (8.2%) and best fluorescence property (Fig. S2†), it will be selected in the following sections.As shown in Fig. 1, the obvious UV-vis absorption band of CDs centered at 257 nm is ascribed to π–π* electron transition of the conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C band. The absorption edge extending to 400 nm indicating the formation of nanocarbon.36 The CDs had emission peak at 420 nm when excited at 340 nm, and showed blue fluorescence under UV light (inset of Fig. 1). Similar to that of fluorescent carbon materials, the CDs showed excitation-dependent fluorescence behavior in Fig. S3A and S3B.† The emission peak of CDs shifted to higher wavelengths accompanied by changed fluorescence intensity as excitation wavelength increased from 310 to 380 nm. It is believed that this phenomenon could be attributed to emissive traps, electronic conjugated structures, and free zigzag sites of the CDs.37
C band. The absorption edge extending to 400 nm indicating the formation of nanocarbon.36 The CDs had emission peak at 420 nm when excited at 340 nm, and showed blue fluorescence under UV light (inset of Fig. 1). Similar to that of fluorescent carbon materials, the CDs showed excitation-dependent fluorescence behavior in Fig. S3A and S3B.† The emission peak of CDs shifted to higher wavelengths accompanied by changed fluorescence intensity as excitation wavelength increased from 310 to 380 nm. It is believed that this phenomenon could be attributed to emissive traps, electronic conjugated structures, and free zigzag sites of the CDs.37
 | ||
| Fig. 1 UV-vis absorption (a) and fluorescence emission spectra (b) (λex = 340 nm) of the CDs. Inset: the image of the CDs dispersion under daylight and UV light (365 nm). | ||
The formation of carbon nanoparticles was first confirmed by TEM measurements. As displayed in Fig. 2A, the CDs were mostly of spherical morphology and dispersed rather evenly with an average diameter of about 2.8 nm (inset of Fig. 2A). In the representative high-resolution TEM (HRTEM) image (Fig. 2B), lattice planes can be clearly identified with a spacing of 0.194 nm which was consistent with the (104) diffraction plane of graphitic carbons (JCPDS 26-1076).38 The corresponding selected-area electron diffraction (SAED) image (inset of Fig. 2B) showed obvious polycrystalline rings which revealed the reasonably good crystallinity of the CDs.
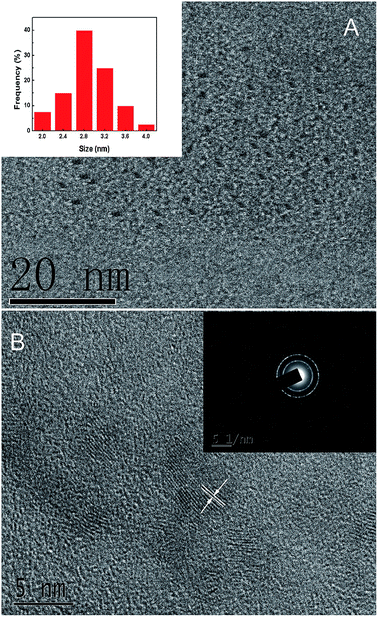 | ||
| Fig. 2 (A) The TEM image of CDs. Inset: the particle size distribution. (B) The HRTEM image of CDs. Inset: the corresponding SAED image. | ||
The surface functional groups of the obtained CDs and L-phenylalaninol were identified by FT-IR spectra. As shown in Fig. 3A, for the L-phenylalaninol (a) and the CDs (b), the peaks between 3000 and 3500 cm−1 are assigned to –OH and –NH2 stretching vibrations. The bands of 2922 and 2854 cm−1 were assigned to –CH2– vibration. The peaks at 1574 and 1446 cm−1 are attributed to the stretching of aromatic CC bonds.39 In Fig. 3Aa, the band at 1436 cm−1 was assigned to the C–N stretching vibrations.40
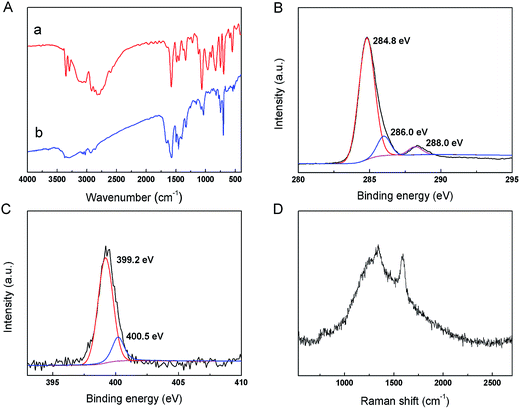 | ||
| Fig. 3 (A) The FT-IR spectra of L-phenylalaninol (a) and CDs (b). (B) High-resolution XPS spectra of C1s and (C) N1s. (D) The Raman spectrum of the CDs. | ||
The surface elements were further identified by XPS. The wide scan XPS spectrum revealed the presence of C1s, N1s, and O1s in CDs. As shown in the high-resolution XPS spectrum of C1s (Fig. 3B), the peak at 284.8 eV could be assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and the peaks at 286.0 and 288.0 eV could be assigned to C–O, C–N, and C
C, and the peaks at 286.0 and 288.0 eV could be assigned to C–O, C–N, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.41,42 The high-resolution N1s spectrum (Fig. 3C) showed two types of N bonds: C–N (399.2 eV) and N–H (400.5 eV),43 which proved that N was doped into the CDs.
O, respectively.41,42 The high-resolution N1s spectrum (Fig. 3C) showed two types of N bonds: C–N (399.2 eV) and N–H (400.5 eV),43 which proved that N was doped into the CDs.
The Raman spectrum (λex = 633 nm) of the prepared CDs (Fig. 3D) exhibited two broad peaks at approximately 1343 and 1592 cm−1, corresponding to the D and G bands, respectively. The D band is associated with the vibrations of carbon atoms with dangling bonds in the termination plane of disordered graphite or glassy carbon, and the G band corresponds to the E2g mode of graphite and is related to the vibration of sp2-hybridized carbon atoms in a two-dimensional hexagonal lattice.44 The D and G bands of the CDs were not very obvious attributing to the fact that high fluorescence of the CDs may disturb the Raman characterization. The intensity ratio of the D and G band (ID/IG) is a measurement of the disorder extent, and the ratio of sp3/sp2 carbon.45 The estimated intensity ratio (ID/IG) of the obtained CDs was ∼1.096, which may result from that some structure defects appeared in the CDs during the L-phenylalaninol thermolysis, such as oxygenated groups (C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in the sp2 carbon site, and then leads to vacant lattice sites and sp3 carbon.46
O) in the sp2 carbon site, and then leads to vacant lattice sites and sp3 carbon.46
UV-vis spectrophotometry was used to prove the presence of β-amino alcohol on the surface of CDs. The basic solution of Cu2+ and CDs showed broad absorption band (500–800 nm) (Fig. S4A†) which was ascribed to d–d transitions.26 Along with the increased concentration of Cu2+, the adsorption increased, and the violet became obviously which was capable to be observed under 1 mmol L−1 Cu2+ (inset of Fig. S4A†). Without the CDs, only blue Cu(OH)2 precipitate was formed and the supernatant showed no UV-vis absorption in 500–800 nm. In addition, 1 mmol L−1 other metal ions (Na+, K+, Ca2+, Mg2+, Fe2+, Fe3+) showed no obvious interference (Fig. S4B†). The UV-vis sensing interface had the LOD of 1 mmol L−1 by naked eyes and the visual detection could be used for Cu2+ assistant analysis (the procedure for UV-vis sensing shown in the ESI†).
The strong blue emission of the prepared fluorescent carbon dots was quenched obviously when copper ions were added into the solution (Fig. S5†). As discussed above, the obtained CDs have the β-amino alcohol moieties at their surface, thus the violet biuret Cu(II) complex was formed by the specific cross-linking with the two β-amino alcohol unites on the surface of CDs. Furthermore, the fluorescence could be quenched by the aggregation of the functionalized CDs during the biuret reaction. These results demonstrate that the analyte (Cu2+)-induced the assembly/aggregation of β-amino alcohol functionalized CDs plays an important role in the selective fluorescent response of the CDs to the Cu2+.
3.2 Factors affecting the fluorescence sensing of Cu2+
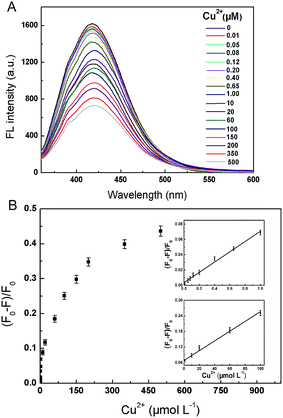
The pH value of the solution is a key factor that affects the sensing interface. The fluorescence of the CDs was investigated under various pH solutions. The fluorescent intensity of the CDs was pH-dependent (Fig. S6A and S6B†). The fluorescence intensity increased evidently with a rise of pH from 2.0 to 8.0 and then increased slowly with a further increase of pH from 8.0 to 12.0. In addition, the fluorescence intensity of the CDs was further investigated in detail under basic conditions. As displayed in Fig. 4A and B, the fluorescence of CDs was strong and relatively stable over the pH values of 8.0–12.0.The quenching efficiencies of Cu2+ towards CDs ((F0 − F)/F0) were investigated under different pH values. The (F0 − F)/F0 increased evidently over the pH range of 2.0 to 10.0, and decreased at pH 12.0 which might ascribe to the hydrolysis of Cu2+ with excess OH− (Fig. S7A†). The (F0 − F)/F0 were further discussed in detail in the pH range of 8.0–12.0 (Fig. S7B†), and reached maximum at the pH of 10.5, which means Cu2+ could react with CDs preferentially and effectively rather than OH− under this condition. In the previous studies,47 the (F0 − F)/F0 are usually affected in basic solutions (pH > 7.0) because the partial hydrolysis of Cu2+ in the basic media inhibits the complex reaction between Cu2+ and the CDs. In this study, this limitation was broken and the quenching efficiencies of Cu2+ towards β-amino alcohol functionalized CDs were still satisfactory in basic environment.
Another key factor that affects the (F0 − F)/F0 is the reaction time. The time-scan spectrum revealed the fast reaction between CDs and Cu2+ which was finished in 10 min (Fig. S8A†). To further verify the quick response, the (F0 − F)/F0 was detected after reacting with CDs for 0–900 s (Fig. S8B†), and reached maximum at 500 s.
To further explore the utility and feasibility of the CDs as fluorescent probe, the impact of the ion strength was investigated. The fluorescence intensity of the CDs was almost not affected even the concentration of sodium chloride was as high as 1.5 mol L−1 (Fig. S9†). All these features make the CDs excellent candidates as an appropriate sensor designed for environmental applications.
3.3 Fluorescence analytical performances for Cu2+ sensing
To examine the selectivity of the CDs, the effects of different kinds of metal ions on the fluorescence quenching were investigated, including Na+, K+, Ca2+, Mg2+, Fe2+, and Fe3+. It can be seen in Fig. S10† that the fluorescent intensity at 420 nm was obviously quenched by Cu2+ of 20 μmol L−1. In contrast, the addition of equal concentration of Fe3+ and Fe2+ and 5 times concentration of other mental ions did not lead to obvious fluorescence quenching. The results implied that the β-amino alcohol moieties on the surface of CDs served as the specific recognition sites of copper ions in the biuret reaction guaranteed the high selectivity of the fluorescent sensor for Cu2+ over other metal ions.The linear response range of the fluorescence sensing interface was measured. As shown in Fig. 5, the fluorescence intensities of the CDs at 420 nm were highly sensitive to Cu2+ and decreased as the concentrations of the analyte increased in the range of 0–500 μmol L−1. A correlation could be established between (F0 − F)/F and the concentration ranges of 0.01–1 and 1–100 μmol L−1 of Cu2+ (Table S2†), and the coefficients were calculated to be 0.993 and 0.994, respectively. The LOD was estimated to be 3.2 nmol L−1 based on the definition of three times the deviation of the blank signal (S = 3σ). Compared with the previous fluorescent CDs sensing systems, the linear range in this work was wider and the LOD was comparable (Table S3†).47–51
3.4 Repeatability and stability
The repeatability of the fluorescence sensing interface was assessed via the determination of Cu2+ by five batches CDs, and the relative standard deviation (RSD) was calculated to be 3.31% (Fig. S11A†). The fluorescence intensity remained about the same after repetitive measurement of 20 times, respectively (Fig. S11B†). And the fluorescence intensity of the CDs decreased 14.28% placed at room temperature for a month. The data above demonstrated the accuracy and practicability of the sensing interface.3.5 Detection of Cu2+ in real water samples
The developed fluorescence sensing method was used to detect Cu2+ in real water samples, including lake water (Xuanwu Lake) and tap water. The studies were carried out with three concentrations of Cu2+ (10, 100, 1000 nmol L−1) spiked in each real sample. Each concentration was done in triplicate and the averages were presented. As shown in Table 1, the measured recoveries and the RSD were satisfactory, indicating that the fluorescence sensing interface performs well for Cu2+ determination in real water.| Samples | Cu2+ content (nmol L−1) | Added Cu2+ (nmol L−1) | Found Cu2+ (nmol L−1) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Lake water | Not detected | 10 | 10.21 | 102.1 | 3.6 |
| 100 | 91.32 | 91.3 | 2.5 | ||
| 1000 | 956.44 | 95.6 | 1.9 | ||
| Tap water | Not detected | 10 | 9.72 | 97.2 | 6.0 |
| 100 | 101.92 | 101.9 | 5.3 | ||
| 1000 | 941.21 | 94.1 | 5.5 |
4. Conclusion
In summary, a facile one-step hydrothermal carbonization of L-phenylalaninol was found to be an effective strategy for the synthesis of β-amino alcohol functionalized CDs without further surface passivation or modification. Different from previous reports, the biuret reaction of β-amino alcohol functionalized CDs with copper ions was first used to fabricate fluorescent sensor under basic conditions. The addition of copper ions could lead to the specific cross-linked formation of the violet complex with the two β-amino alcohol unites on the surface of CDs, and in turn lead to the aggregation of the functionalized CDs and induce fluorescence quenching. This analytical method is simple to operate and quick to response, and the pH range of copper ions detection was broad. Such sensing interface shows sensitive and selective fluorescent signal to copper ions, a wider linear range and lower LOD, and provides new clues for the application of CDs in analytical chemistry.Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81572081, 81273480, and 21175070) and Natural Science Foundation of Jiangsu Province (No. BK20141433). The authors have declared no conflict of interest.References
- X. Y. Xu, R. Ray, Y. L. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS PubMed.
- L. P. Lin, M. C. Rong, F. Luo, D. M. Chen, Y. R. Wang and X. Chen, TrAC, Trends Anal. Chem., 2014, 54, 83–102 CrossRef CAS.
- R. L. Liu, D. Q. Wu, S. H. Liu, K. Koynov, W. Knoll and Q. Li, Angew. Chem., Int. Ed., 2009, 48, 4598–4600 CrossRef CAS PubMed.
- X. M. Lin, G. M. Gao, L. Y. Zheng, Y. W. Chi and G. N. Chen, Anal. Chem., 2014, 86, 1223–1228 CrossRef CAS PubMed.
- H. P. Liu, T. Ye and C. D. Mao, Angew. Chem., Int. Ed., 2007, 46, 6473–6475 CrossRef CAS PubMed.
- M. M. Liu and W. Chen, Nanoscale, 2013, 5, 12558–12564 RSC.
- W. Kwon, G. Lee, S. Do, T. Joo and S. W. Rhee, Small, 2014, 10, 506–513 CrossRef CAS PubMed.
- A. Barati, M. Shamsipur and H. Abdollahi, Biosens. Bioelectron., 2015, 71, 470–475 CrossRef CAS PubMed.
- W. T. Wang, T. Kim, Z. F. Yan, G. S. Zhu, I. Cole, N. T. Nguyen and Q. Li, J. Colloid Interface Sci., 2015, 437, 28–34 CrossRef CAS PubMed.
- X. T. Zheng, A. Ananthanarayanan, K. Q. Luo and P. Chen, Small, 2015, 11, 1620–1636 CrossRef CAS PubMed.
- H. Li, W. Q. Kong, J. Liu, N. Y. Liu, H. Huang, Y. Liu and Z. H. Kang, Carbon, 2015, 91, 66–75 CrossRef CAS.
- J. H. Zhu, M. M. Li, S. P. Liu, Z. F. Liu, Y. F. Li and X. L. Hu, Sens. Actuators, B, 2015, 219, 261–267 CrossRef CAS.
- M. X. Yu, M. Shi, Z. G. Chen, F. Y. Li, X. X. Li, Y. H. Gao, J. Xu, H. Yang, Z. G. Zhou, T. Yi and C. H. Huang, Chem.–Eur. J., 2008, 14, 6892–6900 CrossRef CAS PubMed.
- F. X. Wang, Z. Y. Gu, W. Lei, W. J. Wang, X. F. Xia and Q. L. Hao, Sens. Actuators, B, 2014, 190, 516–522 CrossRef CAS.
- E. Gaggelli, H. Kozlowski, D. Valensin and G. Valensin, Chem. Rev., 2006, 106, 1995–2044 CrossRef CAS PubMed.
- M. Komárek, E. Čadková, V. Chrastný, F. Bordas and J. C. Bollinger, Environ. Int., 2010, 36(1), 138–151 CrossRef PubMed.
- J. R. Brock and G. K. Bielmyer, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2013, 158, 150–158 CrossRef CAS PubMed.
- P. P. Patel and G. K. Bielmyer-Fraser, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2015, 168, 39–47 CrossRef CAS PubMed.
- U. S. EPA, Maximum Contaminant Level Goals and National Primary Drinking Water Regulations for Lead and Copper; Final Rule, Fed. Regist., 1991, 56, 26460–26564 Search PubMed.
- A. P. S. Gonzáles, M. A. Firmino, C. S. Nomura, F. R. P. Rocha, P. V. Oliveira and I. Gaubeur, Anal. Chim. Acta, 2009, 636, 198–204 CrossRef PubMed.
- J. P. Li, L. M. Zhang, G. Wei, Y. Zhang and Y. Zeng, Biosens. Bioelectron., 2015, 69, 316–320 CrossRef CAS PubMed.
- A. Sadollahkhani, A. Hatamie, O. Nur, M. Willander, B. Zargar and I. Kazeminezhad, ACS Appl. Mater. Interfaces, 2014, 6, 17694–17701 CAS.
- J. F. Wu and E. A. Boyle, Anal. Chem., 1997, 69, 2464–2470 CrossRef CAS PubMed.
- Y. S. Liu, Y. N. Zhao and Y. Y. Zhang, Sens. Actuators, B, 2014, 196, 647–652 CrossRef CAS.
- P. M. H. Kroneck, V. Vortisch and P. Hemmerich, Eur. J. Biochem., 1980, 109, 603–612 CrossRef CAS PubMed.
- S. Canepari, V. Carunchio and R. Schina, Polyhedron, 1999, 18, 3263–3267 CrossRef CAS.
- Z. C. Yang, M. Wang, A. M. Yong, S. Y. Wong, X. H. Zhang, H. Tan, A. Y. Chang, X. Li and J. Wang, Chem. Commun., 2011, 47, 11615–11617 RSC.
- Y. F. Wang and A. G. Hu, J. Mater. Chem. C, 2014, 1–18 Search PubMed.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- W. S. Kwon, S. G. Do and S. W. Rhee, RSC Adv., 2012, 2, 11223–11226 RSC.
- R. Liu, D. Wu, S. Liu, K. Koynov, W. Knoll and Q. Li, Angew. Chem., Int. Ed., 2009, 48, 4598–4601 CrossRef CAS PubMed.
- J. Briscoe, A. Marinovic, M. Sevilla, S. Dunn and M. Titirici, Angew. Chem., Int. Ed., 2015, 54, 4463–4468 CrossRef CAS PubMed.
- P. F. Shen and Y. S. Xia, Anal. Chem., 2014, 86, 5323–5329 CrossRef CAS PubMed.
- Y. Q. Dong, R. X. Wang, H. Li, J. W. Shao, Y. W. Chi, X. M. Lin and G. N. Chen, Carbon, 2012, 50, 2810–2815 CrossRef CAS.
- J. Jiang, Y. He, S. Y. Li and H. Cui, Chem. Commun., 2012, 48, 9634–9636 RSC.
- A. Jaiswal, S. S. Ghosh and A. Chattopadhyay, Chem. Commun., 2012, 48, 407–409 RSC.
- H. Huang, C. G. Li, S. J. Zhu, H. L. Wang, C. L. Chen, Z. R. Wang, T. Y. Bai, Z. Shi and S. H. Feng, Langmuir, 2014, 30, 13542–13548 CrossRef CAS PubMed.
- L. Tian, D. Ghosh, W. Chen, S. Pradhan, X. J. Chang and S. W. Chen, Chem. Mater., 2009, 21, 2803–2809 CrossRef CAS.
- X. Wang, K. Qu, B. Xu, J. Ren and X. Qu, J. Mater. Chem., 2011, 21, 2445–2450 RSC.
- X. Zhou, A. Q. Wang, C. F. Yu, S. S. Wu and J. Shen, ACS Appl. Mater. Interfaces, 2015, 7, 11741–11747 CAS.
- M. Zheng, Z. G. Xie, D. Qu, D. Li, P. Du, X. B. Jing and Z. C. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 13242–13247 CAS.
- W. J. Niu, Y. Li, R. H. Zhu, D. Shan, Y. R. Fan and X. J. Zhang, Sens. Actuators, B, 2015, 218, 229–236 CrossRef CAS.
- S. Y. Lu, X. H. Zhao, S. J. Zhu, Y. B. Song and B. Yang, Nanoscale, 2014, 6, 13939–13944 RSC.
- Z. Guo, Z. Q. Zhang, W. Zhang, L. Q. Zhou, H. W. Li, H. M. Wang, C. Andreazza-Vignolle, P. Andreazza, D. X. Zhao, Y. H. Wu, Q. L. Wang, T. Zhang and K. M. Jiang, ACS Appl. Mater. Interfaces, 2014, 6, 20700–20708 CAS.
- H. T. Li, Z. H. Kang, Y. Liu and S. T. Lee, J. Mater. Chem., 2012, 22, 24230–24253 RSC.
- L. B. Li, L. Li, C. Wang, K. Y. Liu, R. H. Zhu, H. Qiang and Y. Q. Lin, Microchim. Acta, 2015, 182, 763–770 CrossRef CAS.
- Y. Q. Dong, R. X. Wang, G. L. Li, C. Q. Chen, Y. W. Chi and G. N. Chen, Anal. Chem., 2012, 84, 6220–6224 CrossRef CAS PubMed.
- Y. F. Sha, J. Y. Lou, S. Z. Bai, D. Wu, B. Z. Liu and Y. Ling, Mater. Res. Bull., 2013, 48, 1728–1731 CrossRef CAS.
- A. W. Zhu, Q. Qu, X. L. Shao, B. Kong and Y. Tian, Angew. Chem., Int. Ed., 2012, 51, 7185–7189 CrossRef CAS PubMed.
- A. D. Zhao, C. Q. Zhao, M. Li, J. S. Ren and X. G. Qu, Anal. Chim. Acta, 2014, 809, 128–133 CrossRef CAS PubMed.
- Q. Qu, A. W. Zhu, X. L. Shao, G. Y. Shi and Y. Tian, Chem. Commun., 2012, 48, 5473–5475 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra24348c |
| This journal is © The Royal Society of Chemistry 2016 |