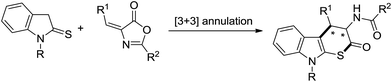
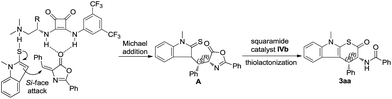
Organocatalyzed enantioselective [3 + 3] annulation for the direct synthesis of conformationally constrained cyclic tryptophan derivatives†
L.-L. Wuab,
Y. Zhengab,
Y.-M. Wangab and
Z.-H. Zhou*ab
aInstitute and State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin 300071, P. R. China. E-mail: z.h.zhou@nankai.edu.cn
bCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071, P. R. China
First published on 18th January 2016
Abstract
An enantioselective formal [3 + 3] annulation of 1-methylindoline-2-thiones and 4-arylmethylideneoxazolin-5(4H)-ones has been developed by the use of an L-tert-leucine-derived bifunctional tertiary amine-squaramide catalyst, which furnished a series of optically active conformationally strained β-branched cyclic tryptophan derivatives in acceptable yields with good to excellent diastereo- and enantioselectivities.
Introduction
Optically active non-proteinogenic amino acids are of great interest due to their significant biological activities, and extensive applications in organic synthesis and new drug discovery.1 Among those valuable amino acids, tryptophan analogues are very attractive motifs owing to their wide range biological significance2 and high versatility as important building blocks of many pharmacologically useful molecules and natural products.3 Accordingly, considerable efforts for the development of efficient synthetic strategies towards these targets have been undertaken and many synthetic methods have been developed to approach this moiety in an asymmetric manner. However, most of the developed methods are mainly focused on the introduction of different substituents on the indole core.4 Currently, conformational constraint has been proven to be a major approach to modify the chemical and biological properties of endogenous bioactive peptide hormones and neurotransmitters.5 Therefore, design and synthesis of conformationally constrained amino acids provides a unique opportunity to obtain new insights into the stereochemical, conformational and topographical requirements of peptide ligand–receptor interactions and for signal transduction. With respect to the conformationally constrained β-substituted tryptophan analogues, reports for the enantioselective route to these compounds have largely relied on the use of chiral starting materials or stoichiometric amounts of chiral inducers.6 To the best of our knowledge, only two asymmetric catalytic version appeared for the synthesis of β-substituted tryptophans. Chen et al. have achieved the synthesis of anti-β-substituted tryptophan derivatives in moderate diastereoselectivities (<44% dr) and good enantioselectivities (41–89% ee) via the copper catalyzed asymmetric Friedel–Crafts alkylation of indoles with nitroacrylates and the subsequent reduction reaction.7 Hou et al. have realized the generation of anti-β-substituted tryptophans with improved diastereo- and enantioselectivity by the reaction of glycine derivatives with sulfonylindoles in the presence of catalyst derived from AgCl and a commercially available chiral monodentate phosphoramidite ligand.8 Recently, organocatalyzed enantioselective tandem processes have become a powerful, fascinating, and highly efficient tool for the rapid assembly of complex structures.9 Undoubtedly, the application of these powerful strategies to the synthesis of novel optically active conformationally strained tryptophan derivatives will be of great importance and highly desirable. Currently, indolin-2-thiones have been proven to be versatile 1,3-dinucleophiles in the organocatalyzed annulation reactions,10 we envisioned that cyclic β-substituted tryptophans could be conveniently accessed via a formal [3 + 3] cyclization between indolin-2-thiones and 4-arylmethylideneoxazolin-5(4H)-ones (Scheme 1). As part of our continued interest in the organocatalyzed cascade reactions,11 herein, we report an efficient and straightforward protocol to the synthesis of conformationally strained cyclic β-substituted tryptophan derivatives via bifunctional squaramide catalyzed asymmetric [3 + 3] cascade reaction of indolin-2-thiones and 4-arylmethylideneoxazolin-5(4H)-ones.Results and discussion
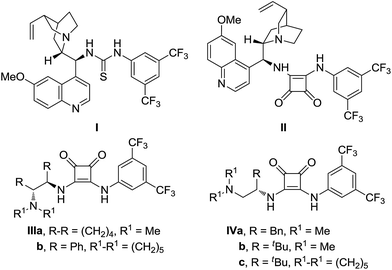
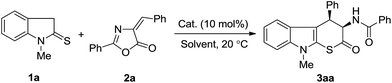
We commenced our study by exposure of 1-methylindoline-2-thione (1a) with (E)-4-benzylidene-2-phenyloxazol-5(4H)-one (2a) in dichloromethane at room temperature in the presence of 10 mol% of various readily available chiral thiourea-12 and squaramide-based13 bidentate hydrogen bond donor catalysts I–IV (Fig. 1).As shown in Table 1, all the tested catalysts, either thiourea or squaramide promoted the model reaction efficiently to generate the corresponding β-branched cyclic tryptophan derivative 3aa in acceptable yields with excellent diastereoselectivities (entries 1–7, >19/1 dr). With regard to the enantioselectivity, the use of quinine-derived thiourea I afforded the desired product 3aa with moderate enantioselectivty (entry 1, 56% ee). A slightly improved ee value was observed for bifunctional squaramide II incorporating the same diamine scaffold as I (entry 2 vs. 1). Further screening of squaramide-based catalysts IIIa,b, and IVa,b that bearing different chiral diamine scaffolds revealed that squaramide IVb, derived from L-tert-leucine, was the promising catalyst for this cascade process and provided product 3aa with an obviously enhanced ee value (entry 6, 91% ee). No superior result was obtained by changing the substituent attached to the nitrogen atom (entry 7).
| Entry | Cat. | Solvent | Time (d) | Yieldb (%) | drc | eed (%) |
|---|---|---|---|---|---|---|
| a Unless otherwise specified, all reactions were carried out by using 1a (0.22 mmol), 2a (0.20 mmol) in the presence of 10 mol% of catalyst in 1.0 mL of solvent at 20 °C.b Isolated yield.c Determined by 1H NMR analysis.d Determined by HPLC analysis using a chiral stationary phase.e The reaction was performed at 40 °C.f 20 mol% of catalyst IVb was loaded.g 1.5 equivalent of 1a was employed.h The reaction was performed in 2 mL of chloroform. | ||||||
| 1 | I | CH2Cl2 | 3 | 43 | >19/1 | 56 |
| 2 | II | CH2Cl2 | 3 | 53 | >19/1 | 64 |
| 3 | IIIa | CH2Cl2 | 3 | 40 | >19/1 | −72 |
| 4 | IIIb | CH2Cl2 | 3 | 47 | >19/1 | −59 |
| 5 | IVa | CH2Cl2 | 3 | 52 | >19/1 | 65 |
| 6 | IVb | CH2Cl2 | 3 | 65 | >19/1 | 91 |
| 7 | IVc | CH2Cl2 | 3 | 68 | >19/1 | 84 |
| 8 | IVb | CHCl3 | 3 | 62 | >19/1 | 93 |
| 9 | IVb | ClCH2CH2Cl | 3 | 49 | >19/1 | 92 |
| 10 | IVb | PhCl | 3 | 47 | >19/1 | 92 |
| 11 | IVb | Toluene | 3 | 48 | >19/1 | 88 |
| 12 | IVb | Et2O | 3 | 38 | >19/1 | 27 |
| 13 | IVb | EtOAc | 3 | 39 | >19/1 | 4 |
| 14 | IVb | CH3CN | 3 | 35 | >19/1 | 2 |
| 15e | IVb | CHCl3 | 2 | 57 | >19/1 | 71 |
| 16f | IVb | CHCl3 | 2 | 60 | >19/1 | 92 |
| 17g | IVb | CHCl3 | 2.5 | 63 | >19/1 | 92 |
| 18h | IVb | CHCl3 | 4 | 58 | >19/1 | 89 |
Having identified squaramide IVb as the optimal catalyst for this cascade transformation, we then turned our attention to the effect of the solvent. The results listed in Table 1 (entries 6, 8–14) clearly indicate that the reaction medium plays an important role on the reaction. In all cases, although excellent diastereoselectivity was observed, the enantioselectivity was highly dependent on the solvent employed. For example, excellent enantioselectivities were obtained by performing the reaction in chlorohydrocarbons, such as methylene chloride, chloroform, 1,2-dichloroethane and chlorobenzene (entries 6, 8–10, 91–93%). Toluene was also found to be a good solvent for this transformation, affording product 3aa with 88% ee (entry 11). The ee value decreased sharply when ether, ethyl acetate or acetonitrile was used as the solvent (entries 12–14), and almost racemic 3aa was obtained in ethyl acetate and acetonitrile (entries 13 and 14). Chloroform proved to be the best solvent giving the highest ee value of 93% among all the tested solvents (entry 8). Attempt to accelerate the reaction rate and improve the yield by conducting the reaction at elevated temperature failed. Raising the temperature to 40 °C indeed accelerated the reaction but led to an obvious decrease in both yield and enantiomeric excess (entry 15). Increasing the catalyst loading (entry 16), tuning the ratio of substrates (entry 17) and performing the reaction in diluted concentration (entry 18) all failed to further improve the yield and enantioselectivity of the reaction.
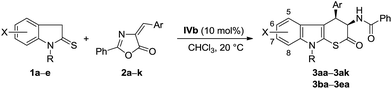
With the optimized reaction conditions in hand, the scope of this [3 + 3] cascade reaction was explored (Table 2). The reaction was found to be tolerant of both electron-deficient and electron-rich 4-arylmethylideneoxazolin-5(4H)-ones (2), delivering the desired cyclic β-substituted tryptophans in acceptable yields (50–67%) with excellent diastereoselectivities (>19/1 dr). With respect to enantioselectivity, the introduction of substituent on the benzene ring resulted in somewhat decrease in ee value. Generally, compared with the results of those substrates bearing electron-withdrawing substituent, some loss of stereocontrol was observed for electron-donating group substituted unsaturated azalactones (entries 2–5 vs. entries 7–10). In the case of bromo-substituted azalactones, the substitution pattern demonstrated a significant impact on the stereochemical outcome of the reaction. The reaction of para-substituted azalactone 2e gave the corresponding product 3ae with 91% ee, while a sharply decreased ee value was obtained for ortho-substituted azalactone 2f (entry 5 vs. entry 6). 4-Arylmethylideneoxazolin-5(4H)-ones 2k bearing a heteroaromatic group was also a suitable reaction partner, generating the corresponding addition/thiolactonization product 3ak in 47% yield with 80% ee (entry 11). Moreover, tolerance to substitution on the indolin-2-thione 1 was also investigated. In all cases, the tandem Michael–thiolactonization reaction took place efficiently to provide the desired syn-β-substituted tryptophans in acceptable yields with excellent diastereo- (>19/1) and good enantioselectivities (75–84% ee) regardless of the nature of the substituents and the substitution pattern (entries 12–14). In addition, N-benzylindolin-2-thione 1e can also be subject to this transformation, giving the corresponding annulation product 3ea in 41% yield with >19/1 dr and 68% ee (entry 15).
| Entry | 3 (X, R, Ar) | Time (d) | Yieldb (%) | drc | eed (%) |
|---|---|---|---|---|---|
| a All reactions were carried out using 1a (0.22 mmol), 2a (0.20 mmol) in chloroform (1.0 mL) at 20 °C in the presence of 10 mol% of catalyst IVb.b Isolated yield.c Determined by 1H NMR analysis.d Determined by HPLC analysis with a chiral stationary phase. | |||||
| 1 | 3aa (H, Me, Ph) | 3 | 62 | >19/1 | 93 |
| 2 | 3ab (H, Me, 4-FC6H4) | 3 | 57 | >19/1 | 87 |
| 3 | 3ac (H, Me, 4-ClC6H4) | 3 | 53 | >19/1 | 81 |
| 4 | 3ad (H, Me, 3-ClC6H4) | 3 | 67 | >19/1 | 85 |
| 5 | 3ae (H, Me, 4-BrC6H4) | 3 | 61 | >19/1 | 91 |
| 6 | 3af (H, Me, 2-BrC6H4) | 3 | 59 | >19/1 | 54 |
| 7 | 3ag (H, Me, 4-MeOC6H4) | 3 | 53 | >19/1 | 73 |
| 8 | 3ah (H, Me, 2-MeOC6H4) | 3 | 50 | >19/1 | 72 |
| 9 | 3ai (H, Me, 3-MeC6H4) | 3 | 57 | >19/1 | 72 |
| 10 | 3aj (H, Me, 2-MeC6H4) | 3 | 59 | >19/1 | 70 |
| 11 | 3ak (H, Me, 2-thienyl) | 3 | 47 | >19/1 | 80 |
| 12 | 3ba (6-F, Me, Ph) | 3 | 53 | >19/1 | 75 |
| 13 | 3ca (6-Br, Me, Ph) | 3 | 54 | >19/1 | 84 |
| 14 | 3da (5-Me, Me, Ph) | 3 | 43 | >19/1 | 80 |
| 15 | 3ea (H, Bn, Ph) | 4 | 41 | >19/1 | 68 |
The relative and absolute configuration of the product 3aa is unequivocally established as 2R,3R by X-ray analysis (Fig. 2), and the remaining configurations are assumed by analogy.14
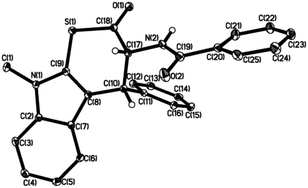 | ||
| Fig. 2 X-ray crystal structure of (R,R)-3aa. Most of the hydrogen atoms have been omitted for clarity. | ||
To account for the observed stereochemical outcome of the reaction, a possible mechanism is proposed for this cascade transformation (Scheme 2). Unsaturated azalactone 2a is fixed and activated by the squaramide moiety via double hydrogen bonding interactions. Then the direct approach of the thioenolate formed by deprotonating of 1-methylindolin-2-thione 1a with the tertiary amine functionality from the Si-face to the double bond of unsaturated azalactone 2a gives the Michael addition intermediate A. Subsequently, thiolactonization of the Michael addition product A with the aid of the bifunctional squaramide catalyst IVb generated the desired product (R,R)-3aa.
Experimental
General methods
All reagents and solvents were commercial grade and purified prior to use when necessary. NMR spectra were acquired on Varian 400 MHz instrumental. Chemical shifts are measured relative to residual solvent peaks as an internal standard set to δ 7.26 and δ 77.0 (CDCl3). Specific rotations were measured on a Perkin-Elmer 341 MC polarimeter. Enantiomeric excesses were determined on a Shimadzu LC-20A instrument (chiral column; mobile phase: hexane/i-PrOH). HRMS was performed on a Varian QFT-ESI instrumental. Melting points were determined on a Taike X-4 melting point apparatus. All temperatures were uncorrected. The racemic samples for HPLC analysis were prepared via triethylamine catalyzed reaction of 1-methylindolin-2-thiones and 4-arylmethylideneoxazolin-5(4H)-ones.General procedure for IVb-catalyzed cascade Michael addition–thiolactonization reaction of 1-methylindolin-2-thiones and 4-arylmethylideneoxazolin-5(4H)-ones
A solution of squaramide catalyst IVb (9 mg, 0.02 mmol, 10 mol%), 1-methylindolin-2-thione (1a, 36 mg, 0.22 mmol) and (E)-4-benzylidene-2-phenyloxazol-5(4H)-one (2a, 50 mg, 0.20 mmol) in chloroform (1 mL) was stirred at 20 °C for three days. After removal of solvent under reduced pressure, the crude product purified through column chromatography on silica gel (200–300 mesh, PE/EtOAc = 8/1) to afford the desired β-branched cyclic tryptophan 3aa (51 mg) as a white solid. The title compounds were fully characterized by 1H NMR, 13C NMR, HRMS and specific rotation data. The enantiomeric excess of the pure products was determined by HPLC analysis using a chiral stationary phase.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-propanol = 70
2-propanol = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 20.14 (minor) and 27.71 min (major).
30, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 20.14 (minor) and 27.71 min (major).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-propanol = 70
2-propanol = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 17.97 (minor) and 24.79 min (major).
30, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 17.97 (minor) and 24.79 min (major).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-propanol = 80
2-propanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 24.07 (minor) and 32.23 min (major).
20, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 24.07 (minor) and 32.23 min (major).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-propanol = 70
2-propanol = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 14.39 (minor) and 23.40 min (major).
30, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 14.39 (minor) and 23.40 min (major).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-propanol = 80
2-propanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 26.45 (minor) and 32.42 min (major).
20, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 26.45 (minor) and 32.42 min (major).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-propanol = 70
2-propanol = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 9.64 (minor) and 22.77 min (major).
30, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 9.64 (minor) and 22.77 min (major).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-propanol = 70
2-propanol = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 33.49 (minor) and 49.36 min (major).
30, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 33.49 (minor) and 49.36 min (major).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-propanol = 70
2-propanol = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 13.37 (minor) and 25.91 min (major).
30, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 13.37 (minor) and 25.91 min (major).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-propanol = 70
2-propanol = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 14.85 (minor) and 19.81 min (major).
30, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 14.85 (minor) and 19.81 min (major).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-propanol = 70
2-propanol = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 8.14 (major) and 17.25 min (minor).
30, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 8.14 (major) and 17.25 min (minor).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-propanol = 70
2-propanol = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 25.35 (minor) and 35.23 min (major).
30, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 25.35 (minor) and 35.23 min (major).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-propanol = 80
2-propanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 22.14 (major) and 72.05 min (minor).
20, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 22.14 (major) and 72.05 min (minor).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-propanol = 70
2-propanol = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 24.82 (minor) and 36.32 min (major).
30, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 24.82 (minor) and 36.32 min (major).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-propanol = 80
2-propanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 12.86 (major) and 25.11 min (minor).
20, flow rate = 1.0 mL min−1, wavelength = 220 nm): Rt = 12.86 (major) and 25.11 min (minor).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-propanol = 70
2-propanol = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, flow rate = 1.0 mL min−1, wavelength = 254 nm): Rt = 17.56 (major) and 46.73 min (minor).
30, flow rate = 1.0 mL min−1, wavelength = 254 nm): Rt = 17.56 (major) and 46.73 min (minor).Conclusions
In conclusion, we have developed an organocatalyzed asymmetric formal [3 + 3] annulation of 1-methylindolin-2-thiones and 4-arylmethylideneoxazolin-5(4H)-ones, which provided a convenient approach to optically active conformationally strained syn-β-branched cyclic tryptophan derivatives. Under the catalysis of chiral squaramide derived from L-tert-leucine, a wide range of substituted 1-methylindolin-2-thiones and 4-arylmethylideneoxazolin-5(4H)-ones tolerated well in this transformation to provide the corresponding biologically significant cyclic tryptophan derivatives in acceptable yield with high levels of diastereo- and enantioselectivity.Acknowledgements
We are grateful to the National Natural Science Foundation of China (No. 21421062), the Key laboratory of Elemento-Organic Chemistry and Collaborative Innovation Center of Chemical Science and Engineering for generous financial support for our programs.Notes and references
- (a) Amino Acids, Peptides, and Proteins in Organic Chemistry, ed. A. B. Hughes, Wiley-VCH, Weinheim, 2009, vol. 1 and 2 Search PubMed; (b) G. C. Barrett and D. T. Elmore, Amino Acids and Peptides, Cambridge University Press, Cambridge, 1998 Search PubMed; (c) F. P. J. T. Rutjes, L. B. Wolf and H. E. Schoemaker, J. Chem. Soc., Perkin Trans. 1, 2000, 4197 RSC.
- (a) Y. Huang, Y. Liu, Y. Liu, H. Song and Q. Wang, Bioorg. Med. Chem., 2016, 24, 462 CrossRef CAS PubMed; (b) Y. Wan, S. Wu, G. Xiao, T. Liu, X. Hou, C. C. P. Guan, X. Yang and H. Fang, Bioorg. Med. Chem., 2015, 23, 1994 CrossRef CAS PubMed; (c) A. P. Podorieszach and H. E. K. Huttunen-Hennelly, Org. Biomol. Chem., 2010, 8, 1679 RSC; (d) S. J. Koopmans, M. Ruis, R. Dekker and M. Korte, Physiol. Behav., 2009, 98, 402 CrossRef CAS PubMed; (e) R. Andersen, M. Leblanc, H. Brastianos, E. Vottero, M. Roberge, G. Mauk and G. Carr, WO 2008052352, 2008Chem. Abstr., 2008, 148, 538426; (f) X. Li, W. Yin, P. V. V. S. Sarma, H. Zhou, J. Ma and J. M. Cook, Tetrahedron Lett., 2004, 45, 8569 CrossRef CAS.
- (a) Y. Feng and G. Chen, Angew. Chem., Int. Ed., 2010, 49, 958 CrossRef CAS PubMed; (b) B. Ma, B. Banerjee, D. N. Litvinov, L. He and S. L. Castle, J. Am. Chem. Soc., 2010, 132, 1159 CrossRef CAS PubMed; (c) W. Hu, F. Zhang, Z. Xu, Q. Liu, Y. Cui and Y. Jia, Org. Lett., 2010, 12, 956 CrossRef CAS PubMed; (d) B. Ma, D. N. Litvinov, L. He, B. Banerjee and S. L. Castle, Angew. Chem., Int. Ed., 2009, 48, 6104 CrossRef CAS PubMed; (e) L. He, L. Yang and S. L. Castle, Org. Lett., 2006, 8, 1165 CrossRef CAS PubMed; (f) D. J. Bentley, A. M. Z. Slawin and C. J. Moody, Org. Lett., 2006, 8, 1975 CrossRef CAS PubMed; (g) A. Zask, J. Kaplan, S. Musto and F. Loganzo, J. Am. Chem. Soc., 2005, 127, 17667 CrossRef CAS PubMed; (h) C. Liu, M. N. Masuno, J. B. MacMillan and T. F. Molinski, Angew. Chem., Int. Ed., 2004, 43, 5951 CrossRef CAS PubMed; (i) R. N. Sonnenschein, J. J. Farias, K. Tenney, S. L. Mooberry, E. Lobkovsky, J. Clardy and P. Crews, Org. Lett., 2004, 6, 779 CrossRef CAS PubMed; (j) C. Chevallier, A. D. Richardson, M. C. Edler, E. Hamel, M. K. Harper and C. M. Ireland, Org. Lett., 2003, 5, 3737 CrossRef CAS PubMed; (k) J. Kobayashi, H. Suzuki, K. Shimbo, K. Takeya and H. Morita, J. Org. Chem., 2001, 66, 6626 CrossRef CAS PubMed; (l) E. Vedejs and C. Kongkittingam, J. Org. Chem., 2001, 66, 7355 CrossRef CAS PubMed; (m) M. K. Renner, Y. C. Shen, X. C. Cheng, P. R. Jensen, W. Frankmoelle, C. A. Kauffman, W. Fenical, E. Lobkovsky and J. Clardy, J. Am. Chem. Soc., 1999, 121, 11273 CrossRef CAS.
- (a) Y.-X. Jia and J.-P. Zhu, Synlett, 2005, 2469 CAS; (b) X. Li, W. Yin, P. V. V. S. Sarma, H. Zhou, J. Ma and J. M. Cook, Tetrahedron Lett., 2004, 45, 8569 CrossRef CAS; (c) S. Gorohovsky, S. Meir, V. Shkoulev, G. Byk and G. Gellerman, Synlett, 2003, 1411 CAS; (d) C. Ma, X. Liu, X. Li, J. Flippen-Anderson, S. Yu and J. M. Cook, J. Org. Chem., 2001, 66, 4525 CrossRef CAS PubMed; (e) W. Wang, C. Xiong, J. Yang and V. J. Hruby, Tetrahedron Lett., 2001, 42, 7717 CrossRef CAS; (f) A. M. Elder and D. H. Rich, Org. Lett., 1999, 1, 1443 CrossRef CAS PubMed; (g) W. J. Drury III, D. Ferraris, C. Cox, B. Young and T. Lectka, J. Am. Chem. Soc., 1998, 120, 11006 CrossRef; (h) R. Liu, P. Zhang, T. Gan and J. M. Cook, J. Org. Chem., 1997, 62, 7447 CrossRef CAS PubMed; (i) Y. Yokoyama, T. Matsumoto and Y. Murakami, J. Org. Chem., 1995, 60, 1486 CrossRef CAS.
- (a) V. J. Hruby, G. Li, C. Haskell-Luevano and M. Shenderovich, Biopolymers, 1997, 43, 219 CrossRef CAS PubMed; (b) V. J. Hruby, Biopolymers, 1993, 31, 1073 CrossRef PubMed; (c) J.-L. Fauchltre and C. Thurieau, Adv. Drug Res., 1992, 23, 127 Search PubMed.
- (a) J. Wang, S. Zhou, D. Lin, X. Ding, H. Jiang and H. Liu, Chem. Commun., 2011, 47, 8355 RSC; (b) S. C. Valdez and J. L. Leighton, J. Am. Chem. Soc., 2009, 131, 14638 CrossRef CAS PubMed; (c) D. Damour, J. P. Pulicani, M. Vuilhorgne and S. Mignani, Synlett, 1999, 786 CrossRef CAS; (d) R. Rodriguez, I. Vinets, A. Diez, M. Rubiralta and E. Giralt, Synth. Commun., 1996, 26, 3029 CrossRef CAS; (e) G. Shapiro, D. Buechler, M. Marzi, K. Schmidt and B. Gomezlor, J. Org. Chem., 1995, 60, 4978 CrossRef CAS; (f) L. Dubois, A. Mehta, E. Tourette and R. H. Dodd, J. Org. Chem., 1994, 59, 434 CrossRef CAS; (g) M. Bruncko and D. Crich, J. Org. Chem., 1994, 59, 4239 CrossRef CAS; (h) L. W. Boteju, K. Wegner, X. H. Qian and V. J. Hruby, Tetrahedron, 1994, 50, 2391 CrossRef CAS; (i) M. Behforouz, H. Zarrinmayeh, M. E. Ogle, T. J. Riehle and F. W. Bell, J. Heterocycl. Chem., 1988, 25, 1627 CrossRef CAS.
- Y. Sui, L. Liu, J.-L. Zhao, D. Wang and Y.-J. Chen, Tetrahedron, 2007, 63, 5173 CrossRef CAS.
- B.-H. Zheng, C.-H. Ding, X.-L. Hou and L.-X. Dai, Org. Lett., 2010, 12, 1688 CrossRef CAS PubMed.
- (a) H. Pellissier, Chem. Rev., 2013, 113, 442 CrossRef CAS PubMed; (b) H. Pellissier, Adv. Synth. Catal., 2012, 354, 237 CrossRef CAS; (c) S. Fustero, M. Sanchez-Rosello and C. del Pozo, Pure Appl. Chem., 2010, 82, 669 CrossRef CAS; (d) D. Enders, C. Grondal and M. R. M. Hüttl, Angew. Chem., Int. Ed., 2007, 46, 1570 CrossRef CAS PubMed.
- (a) X. Chen, J.-Q. Zhang, S.-J. Yin, H.-Y. Li, W.-Q. Zhou and X.-W. Wang, Org. Lett., 2015, 17, 4188 CrossRef CAS PubMed; (b) X. Chen, Z.-H. Qi, S.-Y. Zhang, L.-P. Kong, Y. Wang and X.-W. Wang, Org. Lett., 2015, 17, 42 CrossRef CAS PubMed; (c) S. Chen, J. Pan, Y. Wang and Z. Zhou, Eur. J. Org. Chem., 2014, 7940 CrossRef CAS.
- (a) Y. Zheng, Y. Wang and Z. Zhou, Chem. Commun., 2015, 51, 11652 Search PubMed; (b) H. Wang, L. Wu, Y. Wang and Z. Zhou, RSC Adv., 2015, 5, 42836 RSC; (c) Y. Liu, Q. Wang, Y. Wang, H. Song and Z. Zhou, ChemCatChem, 2014, 6, 2298 CrossRef CAS; (d) L. Wu, Y. Wang, H. Song, L. Tang, Z. Zhou and C. Tang, ChemCatChem, 2014, 6, 649 CrossRef CAS; (e) L. Zhang, Y. Wang, H. Song, Z. Zhou and C. Tang, Eur. J. Org. Chem., 2014, 3163 CrossRef CAS; (f) L. Wu, Y. Wang and Z. Zhou, Tetrahedron: Asymmetry, 2014, 25, 1389 CrossRef CAS; (g) K. Hu, Y. Wang, Z. Zhou and C. Tang, Tetrahedron, 2014, 70, 181 CrossRef CAS; (h) L. Wu, Y. Wang, H. Song, L. Tang, Z. Zhou and C. Tang, Chem.–Asian J., 2013, 8, 2204 CrossRef CAS PubMed; (i) L. Wu, Y. Wang, H. Song, L. Tang, Z. Zhou and C. Tang, Adv. Synth. Catal., 2013, 355, 1053 CrossRef CAS; (j) Y. Liu, Y. Wang, H. Song, Z. Zhou and C. Tang, Adv. Synth. Catal., 2013, 355, 2254 Search PubMed; (k) K. Hu, A. Lu, Y. Wang, Z. Zhou and C. Tang, Tetrahedron: Asymmetry, 2013, 24, 953 CrossRef CAS.
- For reviews on thiourea-based organocatalysis, see: (a) X. Fang and C.-J. Wang, Chem. Commun., 2015, 51, 1185 RSC; (b) O. V. Serdyuk, C. M. Heckel and S. B. Tsogoeva, Org. Biomol. Chem., 2013, 11, 7051 RSC; (c) W.-Y. Siau and J. Wang, Catal. Sci. Technol., 2011, 1, 1298 RSC; (d) B. Han, J.-L. Li, Y.-C. Xiao, S.-L. Zhou and Y.-C. Chen, Curr. Org. Chem., 2011, 15, 4128 CrossRef CAS; (e) Y. Takemoto, Chem. Pharm. Bull., 2010, 58, 593 CrossRef CAS PubMed; (f) S. J. Connon, Chem.–Eur. J., 2006, 12, 5418 CrossRef PubMed.
- For reviews of squaramide-based organocatalysis, see: (a) P. Chauhan, S. Mahajan, U. Kaya, D. Hack and D. Enders, Adv. Synth. Catal., 2015, 357, 253 CrossRef CAS; (b) J. Alemán, A. Parra, H. Jiang and K. A. Jørgensen, Chem.–Eur. J., 2011, 17, 6890 CrossRef PubMed.
- ESI†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1432790. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra24288f |
| This journal is © The Royal Society of Chemistry 2016 |